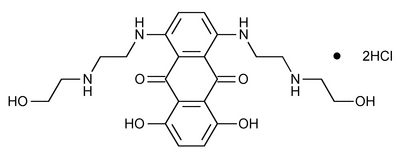
Mitoxantrone Hydrochloride
(mye tox' an trone hye'' droe klor' ide).
9,10-Anthracenedione, 1,4-dihydroxy-5,8-bis[[2-[(2-hydroxyethyl)amino]ethyl]amino]-, dihydrochloride.
1,4-Dihydroxy-5,8-bis[[2-[(2-hydroxyethyl)amino]ethyl]amino]anthraquinone dihydrochloride.
» Mitoxantrone Hydrochloride contains not less than 97.0 percent and not more than 102.0 percent of C22H28N4O6·2HCl, calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers.
Identification, Infrared Absorption  197K
197K .
.
Water, Method I  921
921 :
not more than 6.0%.
:
not more than 6.0%.
Alcohol—
Standard solution—
Transfer 5.0 mL of dehydrated alcohol to a 250-mL volumetric flask, dilute with water to volume, and mix. Transfer 5.0 mL of this solution to a 500-mL volumetric flask, dilute with water to volume, and mix.
Internal standard solution—
Transfer 5.0 mL of n-propyl alcohol to a 250-mL volumetric flask, dilute with water to volume, and mix. Transfer 5.0 mL of this solution to a 500-mL volumetric flask, dilute with water to volume, and mix.
Standard preparation—
Transfer 10.0 mL of the Standard solution to a 25-mL volumetric flask, add 10.0 mL of the Internal standard solution, dilute with water to volume, and mix. This solution contains 0.063 mg of alcohol (C2H5OH) per mL.
Test preparation—
Transfer about 100 mg of Mitoxantrone Hydrochloride, accurately weighed, to a 5-mL volumetric flask, add 2.0 mL of the Internal standard solution, dilute with water to volume, and mix. Sonicate for 2 minutes and shake for 2 minutes, repeating these actions until the specimen is completely dissolved.
Chromatographic system (see Chromatography  621
621 )—
The gas chromatograph is equipped with a flame-ionization detector and a 2-mm × 3-m column that contains 20% phase G1 and 0.1% phase G39 on silanized support S1A. Maintain the column at 50
)—
The gas chromatograph is equipped with a flame-ionization detector and a 2-mm × 3-m column that contains 20% phase G1 and 0.1% phase G39 on silanized support S1A. Maintain the column at 50 for 5 minutes, then increase the temperature at a rate of 30
for 5 minutes, then increase the temperature at a rate of 30 per minute. When 140
per minute. When 140 is reached, maintain that temperature for 20 minutes. Maintain the injection port at 200
is reached, maintain that temperature for 20 minutes. Maintain the injection port at 200 and the detection block at 250
and the detection block at 250 . Use helium as the carrier gas at a flow rate of about 15 mL per minute. Make adjustments if necessary (see System Suitability under Chromatography
. Use helium as the carrier gas at a flow rate of about 15 mL per minute. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ). Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative retention times are about 0.5 for alcohol and 1.0 for n-propyl alcohol, the resolution, R, between the alcohol and the n-propyl alcohol peaks is not less than 6.0, and the tailing factors for the two peaks are not more than 2.0.
). Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative retention times are about 0.5 for alcohol and 1.0 for n-propyl alcohol, the resolution, R, between the alcohol and the n-propyl alcohol peaks is not less than 6.0, and the tailing factors for the two peaks are not more than 2.0.
Procedure—
[note—Use peak areas where peak responses are indicated. ] Separately inject equal volumes (about 1 µL) of the Standard preparation and the Test preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the percentage of alcohol (C2H5OH) in the portion of Mitoxantrone Hydrochloride taken by the formula:
500(C/W)(RU / RS)
in which C is the concentration, in mg per mL, of alcohol (C2H5OH) in the Standard preparation; W is the weight, in mg, of Mitoxantrone Hydrochloride taken; and RU and RS are the ratios of the response of the alcohol peak to that of the n-propyl alcohol peak obtained from the Test preparation and the Standard preparation, respectively: not more than 1.5% is found.
Heavy metals  231
231 —
Proceed as directed under Method II, except in the Procedure to filter the final solutions through a suitable acid-resistant membrane filter of 0.22 µm or finer porosity, instead of viewing them over a dark surface: the precipitate on the filter obtained from the Test Preparation is not darker than that obtained from the Standard Preparation. The limit is 0.002%.
—
Proceed as directed under Method II, except in the Procedure to filter the final solutions through a suitable acid-resistant membrane filter of 0.22 µm or finer porosity, instead of viewing them over a dark surface: the precipitate on the filter obtained from the Test Preparation is not darker than that obtained from the Standard Preparation. The limit is 0.002%.
Chromatographic purity—
Using the chromatogram of the Assay preparation obtained as directed in the Assay, calculate the percentage of each impurity in the Mitoxantrone Hydrochloride taken by the formula:
100(ri / rs)
in which ri is the response of any individual peak, other than the main mitoxantrone peak, and rs is the sum of the responses of all the peaks in the chromatogram, including that of the main mitoxantrone peak: not more than 1.0% of any individual impurity and not more than 2.0% of total impurities is found.
Assay—
Sodium 1-heptanesulfonate solution—
Dissolve 22.0 g of sodium 1-heptanesulfonate in about 150 mL of water, pass through a suitable filter having a 0.5-µm or finer porosity, and transfer the filtrate to a 250-mL volumetric flask. Wash the filter with about 50 mL of water, adding the filtrate to the 250-mL volumetric flask. Add 32.0 mL of glacial acetic acid to the volumetric flask, dilute with water to volume, and mix.
Mobile phase—
Prepare a suitable degassed mixture of water, acetonitrile, and Sodium 1-heptanesulfonate solution (750:250:25). Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
System suitability solution—
Prepare a solution of USP Mitoxantrone System Suitability Mixture RS in a suitable volume of Mobile phase to obtain a solution containing about 0.2 mg of 8-amino-1,4-dihydroxy-5[[2-[(2-hydroxyethyl)amino]ethyl]amino]-9,10-anthracenedione hydrochloride (mitoxantrone related compound A) and 0.1 mg of mitoxantrone hydrochloride per mL.
Standard preparation—
Transfer about 20 mg of USP Mitoxantrone Hydrochloride RS, accurately weighed, to a 50-mL volumetric flask, add 40 mL of Mobile phase, and dissolve by sonicating for about 5 minutes. Cool to room temperature, dilute with Mobile phase to volume, and mix.
Assay preparation—
Transfer about 20 mg of Mitoxantrone Hydrochloride, accurately weighed, to a 50-mL volumetric flask, add 40 mL of Mobile phase, and dissolve by sonicating for about 5 minutes. Cool to room temperature, dilute with Mobile phase to volume, and mix.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 254-nm detector and a 3.9-mm × 30-cm column that contains packing L11. The flow rate is about 3 mL per minute. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.7 for mitoxantrone and 1.0 for mitoxantrone related compound A; the resolution, R, between mitoxantrone and mitoxantrone related compound A is not less than 3.0; and the tailing factor for the mitoxantrone peak is not more than 2.0. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the capacity factor, k¢, for mitoxantrone is not less than 3.5; and the relative standard deviation for replicate injections is not more than 2.0%.
)—
The liquid chromatograph is equipped with a 254-nm detector and a 3.9-mm × 30-cm column that contains packing L11. The flow rate is about 3 mL per minute. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.7 for mitoxantrone and 1.0 for mitoxantrone related compound A; the resolution, R, between mitoxantrone and mitoxantrone related compound A is not less than 3.0; and the tailing factor for the mitoxantrone peak is not more than 2.0. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the capacity factor, k¢, for mitoxantrone is not less than 3.5; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 50 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the areas for the major peaks. [note—After use, wash the column with a mixture of acetonitrile and water (50:50), and store in this mixture. ] Calculate the quantity, in mg, of C22H28N4O6·2HCl in the portion of Mitoxantrone Hydrochloride taken by the formula:
50C(rU / rS)
in which C is the concentration, in mg per mL, of anhydrous mitoxantrone hydrochloride in the Standard preparation, as determined from the content of USP Mitoxantrone Hydrochloride RS corrected for the water content determined by a titrimetric water determination; and rU and rS are the mitoxantrone peak areas obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ahalya Wise, M.S.
Senior Scientific Liaison 1-301-816-8161 |
(SM12010) Monographs - Small Molecules 1 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3938