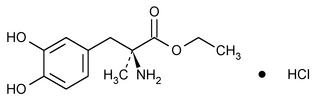
Methyldopate Hydrochloride
(meth'' il doe' pate hye'' droe klor' ide).
l-Tyrosine, 3-hydroxy-
l-3-(3,4-Dihydroxyphenyl)-2-methylalanine ethyl ester hydrochloride
» Methyldopate Hydrochloride contains not less than 98.0 percent and not more than 101.0 percent of C12H17NO4·HCl, calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers. Store at 25 , excursions permitted between 15
, excursions permitted between 15 and 30
and 30 .
.
Identification—
B:
Ultraviolet Absorption  197U
197U —
—
Solution:
50 µg per mL.
Medium:
0.1 N hydrochloric acid.
Absorptivities at 280 nm, calculated on the dried basis, do not differ by more than 3.0%.
C:
It responds to Identification test C under Methyldopa.
D:
It responds to the tests for Chloride  191
191 , except that on the addition of the slight excess of 6 N ammonium hydroxide a brown precipitate is formed.
, except that on the addition of the slight excess of 6 N ammonium hydroxide a brown precipitate is formed.
Specific rotation  781S
781S :
between
:
between  13.5
13.5 and
and  14.9
14.9 (
(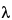 = 405 nm).
= 405 nm).
Test solution:
40 mg per mL, in 0.1 N hydrochloric acid.
pH  791
791 :
between 3.0 and 5.0, in a solution (1 in 100).
:
between 3.0 and 5.0, in a solution (1 in 100).
Loss on drying  731
731 —
Dry it at 100
—
Dry it at 100 and at a pressure not exceeding 5 mm of mercury for 2 hours: it loses not more than 0.5% of its weight.
and at a pressure not exceeding 5 mm of mercury for 2 hours: it loses not more than 0.5% of its weight.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Heavy metals, Method II  231
231 :
0.001%.
:
0.001%.
Assay—
Mobile solvent—
Prepare a suitable solution of 0.02 M monobasic sodium phosphate and 0.015 M phosphoric acid in a water and methanol solution (approximately 15.5:4.5) such that the retention time of methyldopate hydrochloride is approximately 6.5 minutes.
Standard preparation—
Dissolve an accurately weighed quantity of USP Methyldopate Hydrochloride RS in the Mobile solvent to obtain a solution containing about 1 mg per mL.
Assay preparation—
Transfer about 50 mg of Methyldopate Hydrochloride, accurately weighed, to a 50-mL volumetric flask, dissolve in and dilute with Mobile solvent to volume.
Procedure—
Introduce separately 20-µL portions of the Assay preparation and the Standard preparation into a high-pressure liquid chromatograph (see Chromatography  621
621 ) operated at 25
) operated at 25 , by means of a suitable microsyringe or sampling valve, adjusting the operating parameters such that the peak obtained with the Standard preparation is 100% full-scale. Typically, the apparatus is fitted with a 4-mm × 30-cm column that contains packing L1, is equipped with an UV detector capable of monitoring absorption at 280 nm and a suitable recorder, and is capable of operating at a column pressure between 700 and 1700 psi. In a suitable chromatogram, three replicate injections of the Standard preparation show a relative standard deviation of not more than 1.5%. Determine the peak areas, at equivalent retention times, obtained with the Assay preparation and the Standard preparation, and calculate the quantity, in mg, of C12H17NO4·HCl in the portion of Methyldopate Hydrochloride taken by the formula:
, by means of a suitable microsyringe or sampling valve, adjusting the operating parameters such that the peak obtained with the Standard preparation is 100% full-scale. Typically, the apparatus is fitted with a 4-mm × 30-cm column that contains packing L1, is equipped with an UV detector capable of monitoring absorption at 280 nm and a suitable recorder, and is capable of operating at a column pressure between 700 and 1700 psi. In a suitable chromatogram, three replicate injections of the Standard preparation show a relative standard deviation of not more than 1.5%. Determine the peak areas, at equivalent retention times, obtained with the Assay preparation and the Standard preparation, and calculate the quantity, in mg, of C12H17NO4·HCl in the portion of Methyldopate Hydrochloride taken by the formula:
50C(AU / AS)
in which C is the concentration, in mg per mL, of USP Methyldopate Hydrochloride RS in the Standard preparation; and AU and AS are the peak areas obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Sujatha Ramakrishna, Ph.D.
Senior Scientific Liaison 1-301-816-8349 |
(SM22010) Monographs - Small Molecules 2 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3874
Pharmacopeial Forum: Volume No. 29(5) Page 1534