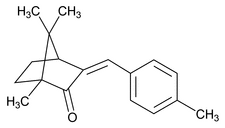
Enzacamene
(en'' za kam' een).
» Enzacamene contains not less than 98.0 percent and not more than 102.0 percent of C18H22O, calculated on the dried basis.
Packaging and storage—
Preserve in tight containers.
USP Reference standards  11
11 —
—
USP Methyl Benzylidene Camphor RS
Identification—
B:
Ultraviolet Absorption  197U
197U —
—
Solution:
10 µg per mL.
Medium:
methanol.
Absorptivities at the wavelength of maximum absorbance, about 299 nm, calculated on the dried basis, do not differ by more than 3.0%.
Melting range, Class Ia  741
741 :
between 66
:
between 66 and 68
and 68 .
.
Loss on drying  731
731 —
Dry it in vacuum at 50
—
Dry it in vacuum at 50 to constant weight: it loses not more than 0.2% of its weight.
to constant weight: it loses not more than 0.2% of its weight.
Related compounds—
Standard solution—
Use the Standard preparation prepared as directed in the Assay.
Test solution—
Use the Assay preparation.
Chromatographic system—
Proceed as directed in the Assay.
Procedure—
Separately inject equal volumes (about 1 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the percentage of each impurity in the portion of Enzacamene taken by the formula:
100(ri / rS)
in which ri is the peak response for each impurity obtained from the Test solution; and rS is the peak response of enzacamene obtained from the Standard solution: not more than 0.02% of camphor is found; not more than 0.1% of any other individual impurity is found; and not more than 0.5% of total impurities is found.
Assay—
Standard preparation—
Dissolve accurately weighed quantities of camphor and USP Methyl Benzylidene Camphor RS in dichloromethane, and dilute quantitatively, and stepwise if necessary, with dichloromethane to obtain a solution having a known concentration of about 250 µg of each per mL.
Assay preparation—
Transfer about 25 mg of Enzacamene, accurately weighed, to a 100-mL volumetric flask, and dissolve in and dilute with dichloromethane to volume.
Chromatographic system (see Chromatography  621
621 )—
The gas chromatograph is equipped with a flame-ionization detector and a 0.32-mm × 30-m fused-silica capillary column coated with a 0.25-µm layer of phase G1. The carrier gas is helium flowing at a rate of about 1.2 mL per minute. The chromatograph is programmed as follows. Initially the temperature of the column is equilibrated at 100
)—
The gas chromatograph is equipped with a flame-ionization detector and a 0.32-mm × 30-m fused-silica capillary column coated with a 0.25-µm layer of phase G1. The carrier gas is helium flowing at a rate of about 1.2 mL per minute. The chromatograph is programmed as follows. Initially the temperature of the column is equilibrated at 100 , then 5 minutes after injection the temperature is increased at a rate of 10
, then 5 minutes after injection the temperature is increased at a rate of 10 per minute to 230
per minute to 230 , and maintained at 230
, and maintained at 230 for 10 minutes. The temperature is then increased at a rate of 10
for 10 minutes. The temperature is then increased at a rate of 10 per minute to 300
per minute to 300 , and maintained at 300
, and maintained at 300 until the end of the procedure. The injection port temperature is maintained at 295
until the end of the procedure. The injection port temperature is maintained at 295 , and the detector is maintained at 305
, and the detector is maintained at 305 . Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative retention times are about 0.2 for camphor and 1.0 for enzacamene; the tailing factor is not more than 1.0; and the relative standard deviation for replicate injections is not more than 2.0%.
. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative retention times are about 0.2 for camphor and 1.0 for enzacamene; the tailing factor is not more than 1.0; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 1 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of C18H22O in the portion of Enzacamene taken by the formula:
100C(rU / rS)
in which C is the concentration, in mg per mL, of USP Methyl Benzylidene Camphor RS in the Standard preparation; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Feiwen Mao, M.S.
Senior Scientific Liaison 1-301-816-8320 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3049