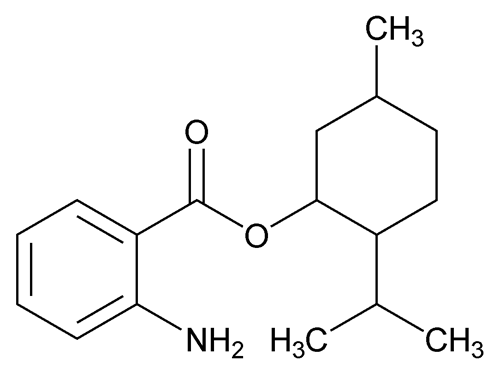
Meradimate
(mer ad' i mate).
» Meradimate contains not less than 95.0 percent and not more than 105.0 percent of C17H25NO2.
Packaging and storage—
Preserve in tight containers.
Identification—
B:
Ultraviolet Absorption  197U
197U —
—
Solution:
5.0 µg per mL.
Medium:
alcohol.
Absorptivities, calculated on the as-is basis, do not differ by more than 3.0%.
Refractive index  831
831 :
between 1.540 and 1.544 at 20
:
between 1.540 and 1.544 at 20 .
.
Acidity—
Transfer 50 mL of alcohol to a suitable container, add 1 mL of phenolphthalein TS, and add sufficient 0.1 N sodium hydroxide to obtain a persistent pink color. Transfer 50 mL of this solution to a suitable container, add about 5.0 mL of Meradimate, accurately measured, mix, and titrate with 0.1 N sodium hydroxide: not more than 0.2 mL of titrant per mL of Meradimate is necessary.
Chromatographic purity—
Test solution—
Use the Assay preparation.
Chromatographic system—
Proceed as directed in the Assay. To evaluate the system suitability requirements, use the Standard preparation prepared as directed in the Assay.
Procedure—
Inject a volume (about 1 µL) of the Test solution into the chromatograph, record the chromatogram, and measure all of the peak responses. Calculate the percentage of each impurity in the portion of Meradimate taken by the formula:
100(ri / rs)
in which ri is the peak response for each impurity; and rs is the sum of the responses of all the peaks: not more than 0.1% of any individual impurity is found; and not more than 2.0% of total impurities is found.
Assay—
Standard preparation—
Dissolve an accurately weighed quantity of USP Meradimate RS in tert-butyl methyl ether, and dilute quantitatively, and stepwise if necessary, with tert-butyl methyl ether to obtain a solution having a known concentration of about 20.0 mg per mL.
Assay preparation—
Transfer about 2 g of Meradimate, accurately weighed, to a 100-mL volumetric flask, dilute with tert-butyl methyl ether to volume, and mix.
Chromatographic system (see Chromatography  621
621 )—
The gas chromatograph is equipped with a flame-ionization detector and a 0.32-mm × 25-m column coated with a 0.1-µm film of G1. The carrier gas is helium, flowing at a rate of about 6 mL per minute. The split ratio is 30:1. [note—The split ratio can be modified in order to optimize performance. ] The chromatograph is programmed as follows. Initially the temperature of the column is equilibrated at 60
)—
The gas chromatograph is equipped with a flame-ionization detector and a 0.32-mm × 25-m column coated with a 0.1-µm film of G1. The carrier gas is helium, flowing at a rate of about 6 mL per minute. The split ratio is 30:1. [note—The split ratio can be modified in order to optimize performance. ] The chromatograph is programmed as follows. Initially the temperature of the column is equilibrated at 60 , then the temperature is increased at a rate of 8
, then the temperature is increased at a rate of 8 per minute to 240
per minute to 240 , and is maintained at 240
, and is maintained at 240 for 10 minutes. The injection port temperature is maintained at 240
for 10 minutes. The injection port temperature is maintained at 240 , and the detector temperature is maintained at 260
, and the detector temperature is maintained at 260 . Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the resolution, R, between meradimate and any other peak is not less than 1.0; and the relative standard deviation for replicate injections is not more than 2.0%.
. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the resolution, R, between meradimate and any other peak is not less than 1.0; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 1 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of C17H25NO2 in the portion of Meradimate taken by the formula:
100C(rU / rS)
in which C is the concentration, in mg per mL, of USP Meradimate RS in the Standard preparation; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Feiwen Mao, M.S.
Senior Scientific Liaison 1-301-816-8320 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3809
Pharmacopeial Forum: Volume No. 34(1) Page 100