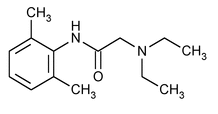
Lidocaine
C14H22N2O 234.34
Acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl)-;
2-(Diethylamino)-2¢,6¢-acetoxylidide

 [137-58-6].
[137-58-6].
(lye' doe kane).
C14H22N2O 234.34
Acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl)-;
2-(Diethylamino)-2¢,6¢-acetoxylidide
DEFINITION
Lidocaine contains NLT 97.5% and NMT 102.5% of C14H22N2O.
IDENTIFICATION
• A. Infrared Absorption  197K
197K :
Previously dried in vacuum over silica gel for 24 h
:
Previously dried in vacuum over silica gel for 24 h
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
Solution A:
Water and glacial acetic acid (930:50). Adjust with 1 N sodium hydroxide to a pH of 3.40.
Mobile phase:
Acetonitrile and Solution A (1:4), so that the retention time of lidocaine is 4–6 min
Standard solution:
Dissolve 85 mg of USP Lidocaine RS, with warming if necessary, in 0.5 mL of 1 N hydrochloric acid in a 50-mL volumetric flask. Dilute with Mobile phase to volume.
System suitability stock solution:
220 µg/mL of methylparaben in Mobile phase
System suitability solution:
Mix 2 mL of System suitability stock solution and 20 mL of Standard solution.
Sample solution:
Dissolve 85 mg of Lidocaine, with warming if necessary, in 0.5 mL of 1 N hydrochloric acid in a 50-mL volumetric flask. Dilute with Mobile phase to volume.
Chromatographic system
Mode:
LC
Detector:
UV 254 nm
Column:
3.9-mm × 30-cm; packing L1
Flow rate:
1.5 mL/min
Injection size:
20 µL
System suitability
Samples:
Standard solution and System suitability solution
Suitability requirements
Resolution:
NLT 3.0 between lidocaine and methylparaben, System suitability solution
Relative standard deviation:
NMT 1.5%, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C14H22N2O in the portion of Lidocaine taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Lidocaine RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Lidocaine in the Sample solution (mg/mL) |
Acceptance criteria:
97.5%–102.5%
IMPURITIES
Inorganic Impurities
• Residue on Ignition  281
281 :
NMT 0.1%
:
NMT 0.1%
• Chloride and Sulfate, Chloride  221
221 :
Dissolve 1.0 g in a mixture of 3 mL of 2 N nitric acid and 12 mL of water, and add 1 mL of silver nitrate TS: the turbidity does not exceed that produced by 50 µL of 0.020 N hydrochloric acid (0.0035%).
:
Dissolve 1.0 g in a mixture of 3 mL of 2 N nitric acid and 12 mL of water, and add 1 mL of silver nitrate TS: the turbidity does not exceed that produced by 50 µL of 0.020 N hydrochloric acid (0.0035%).
• Chloride and Sulfate, Sulfate  221
221 :
Dissolve 100 mg in a mixture of 1 mL of 2 N nitric acid and 10 mL of water. Filter if necessary, and add 1 mL of barium chloride TS. The turbidity does not exceed that produced by 0.10 mL of 0.020 N sulfuric acid (NMT 0.1%).
:
Dissolve 100 mg in a mixture of 1 mL of 2 N nitric acid and 10 mL of water. Filter if necessary, and add 1 mL of barium chloride TS. The turbidity does not exceed that produced by 0.10 mL of 0.020 N sulfuric acid (NMT 0.1%).
• Heavy Metals, Method I  231
231
Test preparation:
1.0 g
Analysis:
Dissolve the Test preparation in a mixture of 2 mL of 3 N hydrochloric acid and 10 mL of water. Evaporate on a steam bath to dryness, and dissolve the residue in 25 mL of water.
Acceptance criteria:
20 ppm
SPECIFIC TESTS
• Melting Range or Temperature  741
741 :
66
:
66 –69
–69
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed containers. Store at room temperature.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Mary S. Waddell
Scientific Liaison 1-301-816-8124 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3681
Pharmacopeial Forum: Volume No. 36(5) Page 1191