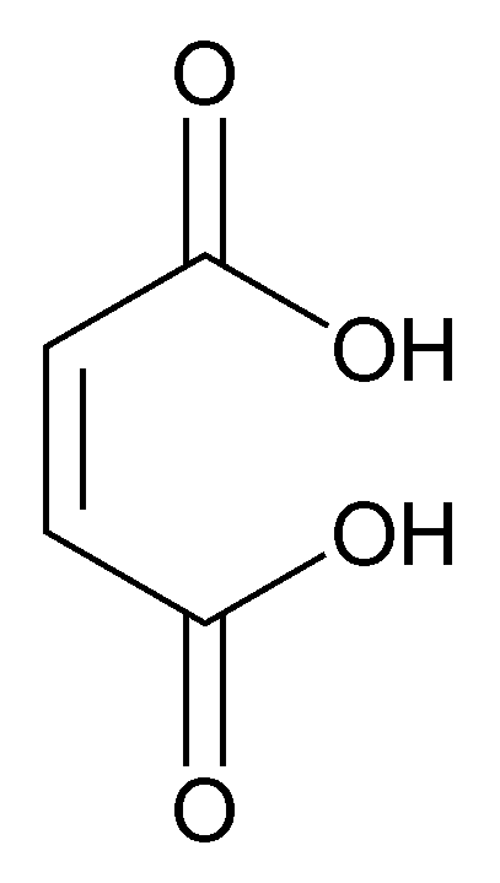
Maleic Acid
(ma lee' ik as' id).
DEFINITION
Maleic Acid contains NLT 99.0% and NMT 101.0% of C4H4O4, calculated on the anhydrous basis.
IDENTIFICATION
• A. Procedure
Analysis:
Dissolve 500 mg of Maleic Acid in 10 mL of water.
Acceptance criteria:
The pH of the solution is less than 2.
• B.
The principal spot from Sample solution B corresponds in color, size, and RF value to that from Standard solution A, as obtained in the procedure for Limit of Fumaric Acid.
ASSAY
• Procedure
Sample solution:
10 mg/mL of Maleic Acid
Analysis:
Titrate the Sample solution with 1 N sodium hydroxide VS, using phenolphthalein TS as the indicator. Each mL of 1 N sodium hydroxide is equivalent to 58.04 mg of C4H4O4.
Acceptance criteria:
99.0%–101.0% on the anhydrous basis
IMPURITIES
Inorganic Impurities
• Residue on Ignition  281
281 :
NMT 0.1%, determined on a 1.0-g portion
:
NMT 0.1%, determined on a 1.0-g portion
• Limit of Iron
Solution A:
Dissolve 9.7 g of potassium thiocyanate in 100 mL of water.
Diluted standard iron solution:
Immediately before use, dilute 1 volume of Standard Iron Solution, prepared as directed in Iron  241
241 , with 9 volumes of water. [Note—This solution contains the equivalent of 1 µg/mL of iron. ]
, with 9 volumes of water. [Note—This solution contains the equivalent of 1 µg/mL of iron. ]
Standard solution:
Add 6 mL of water to 5 mL of Diluted standard iron solution. Add 1 mL of diluted hydrochloric acid and 0.05 mL of bromine TS. After 5 min, remove the excess of bromine with the aid of a current of air, add 3 mL of Solution A, and shake well.
Sample solution:
Dissolve 1 g of Maleic Acid in 10 mL of water. Add 2 mL of diluted hydrochloric acid and 0.05 mL of bromine TS. After 5 min, remove the excess of bromine with the aid of a current of air, add 3 mL of Solution A, and shake well.
Analysis:
Allow the Standard solution and Sample solution to stand for 5 min.
Acceptance criteria:
Any red color in the Sample solution is not more intense than that in the Standard solution (NMT 5 ppm).
• Heavy Metals, Method II  231
231
Test preparation:
Transfer 1.0 g of Maleic Acid to a quartz crucible, and add 0.5 g of magnesium oxide. Ignite the crucible to dull redness until a homogeneous white or grayish-white mass is obtained. Ignite at 800 for 1 h, cool, and dissolve the residue by adding two 5-mL portions of diluted hydrochloric acid. Add 0.1 mL of phenolphthalein TS, then add ammonium hydroxide until a pink color is obtained. Cool, add glacial acetic acid until the solution is decolorized, then add 0.5 mL of glacial acetic acid in excess, and dilute with water to 20.0 mL.
for 1 h, cool, and dissolve the residue by adding two 5-mL portions of diluted hydrochloric acid. Add 0.1 mL of phenolphthalein TS, then add ammonium hydroxide until a pink color is obtained. Cool, add glacial acetic acid until the solution is decolorized, then add 0.5 mL of glacial acetic acid in excess, and dilute with water to 20.0 mL.
Standard solution:
To 0.5 g of magnesium oxide add 1.0 mL of Standard Lead Solution, and evaporate to dryness at 105 for 1 h. Following the procedure described for preparation of the Test preparation, ignite, dissolve in diluted hydrochloric acid, add ammonia and then acetic acid, and dilute with water to 20.0 mL.
for 1 h. Following the procedure described for preparation of the Test preparation, ignite, dissolve in diluted hydrochloric acid, add ammonia and then acetic acid, and dilute with water to 20.0 mL.
Blank:
Water and Test preparation (10:2)
Analysis:
To 12 mL of the Test preparation add 2.0 mL of pH 3.5 Acetate Buffer, mix, add to 1.2 mL of thioacetamide–glycerin base TS, and mix immediately.
To 10 mL of the Standard solution add 2.0 mL of the Test preparation and 2.0 mL of pH 3.5 Acetate Buffer, mix, add 1.2 mL of thioacetamide–glycerin base TS, and mix immediately.
Compared to the Blank, the solution from the Standard solution shows a light brown color. Dilute each of the solutions from the Test preparation and Standard solution with water to 50 mL. Allow to stand for 2 min, and view downward over a white surface.
Acceptance criteria:
The color of the solution from the Test preparation is not darker than that of the solution from the Standard solution (NMT 10 ppm).
Organic Impurities
• Procedure: Limit of Fumaric Acid
Adsorbent:
0.25-mm layer of chromatographic silica gel mixture
Standard solution A:
2 mg/mL of USP Maleic Acid RS in acetone
Standard solution B:
1.5 mg/mL of USP Fumaric Acid RS in acetone
System suitability solution:
Equal volumes of Standard solution A and Standard solution B
Sample solution A:
100 mg/mL of Maleic Acid in acetone
Sample solution B:
2 mg/mL of Maleic Acid in acetone from Sample solution A
Developing solvent system:
Heptane, butanol, chloroform, and anhydrous formic acid (44:36:16:16)
Application volume:
10 µL for the System suitability solution and 5 µL each for Standard solution A, Standard solution B, Sample solution A, and Sample solution B
Analysis
Samples:
Standard solution A, Standard solution B, System suitability solution, Sample solution A, and Sample solution B
Proceed as directed in Chromatography  621
621 , Thin-Layer Chromatography, using an unsaturated chamber. Dry the plate at 100
, Thin-Layer Chromatography, using an unsaturated chamber. Dry the plate at 100 for 15 min, and examine the plate under short-wavelength UV light at 254 nm.
for 15 min, and examine the plate under short-wavelength UV light at 254 nm.
Acceptance criteria:
The chromatogram from the System suitability solution exhibits two clearly separated spots, and any spot corresponding to fumaric acid in the chromatogram from Sample solution A does not exceed, in size or intensity, the principal spot in the chromatogram from Standard solution B (NMT 1.5% of fumaric acid).
SPECIFIC TESTS
• Color and Clarity of Solution
Dilute hydrochloric acid solution:
Dilute 27.5 mL of hydrochloric acid with water to 1000 mL.
Reference solution:
Mix 2.4 mL of ferric chloride CS and 0.6 mL of cobaltous chloride CS with Dilute hydrochloric acid solution to make 10 mL. Dilute 5 mL of this solution with Dilute hydrochloric acid solution to make 100 mL.
Sample solution:
100 mg/mL of Maleic Acid
Analysis:
Place the Reference solution and the Sample solution in matched color-comparison tubes, and compare the solutions by viewing them downward against a white surface (see Color and Achromicity  631
631 ).
).
Acceptance criteria:
The Sample solution is clear and not more intensely colored than the Reference solution.
• Water Determination, Method I  921
921 :
NMT 2.0%
:
NMT 2.0%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight glass containers, protected from light. Store at room temperature.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Robert H. Lafaver, M.S.
Scientific Liaison 1-301-816-8335 |
(EXC2010) Monographs - Excipients |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 1850
Pharmacopeial Forum: Volume No. 36(4) Page 948