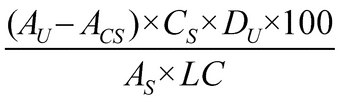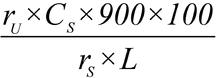Isotretinoin Capsules
» Isotretinoin Capsules contain not less than 90.0 percent and not more than 110.0 percent of the labeled amount of isotretinoin (C20H28O2).
[Caution—Isotretinoin is teratogenic. Avoid inhalation and skin contact.
]
Packaging and storage—
Preserve in tight containers, protected from light. Store at controlled room temperature, in a dry place.
Labeling—
When more than one Dissolution test is given, the labeling states the test used only if Test 1 is not used.
Identification—
The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay.
Dissolution  711
711 —
[Caution—Carry out all the tests under subdued light and use low-actinic glassware.
]
—
[Caution—Carry out all the tests under subdued light and use low-actinic glassware.
]
test 1—
Medium—
stage 1:
simulated gastric fluid with pepsin, prepared freshly and purged with nitrogen.
stage 2:
0.13 N sodium hydroxide, prepared by transferring 5 g of sodium hydroxide to a 1-L volumetric flask and dissolving in and diluting with water to volume. Prepare fresh, and purge with nitrogen.
Apparatus
(see Disintegration  701
701 )—No disks; the apparatus is adjusted so that the bottom of the basket-rack assembly descends to 1.0 ± 0.1 cm from the inside bottom surface of the vessel on the downward stroke; the 10-mesh stainless steel cloth in the basket-rack assembly is replaced with a 40-mesh stainless steel cloth; a 10-mesh stainless-steel cloth is fitted to the top of the basket-rack assembly.
)—No disks; the apparatus is adjusted so that the bottom of the basket-rack assembly descends to 1.0 ± 0.1 cm from the inside bottom surface of the vessel on the downward stroke; the 10-mesh stainless steel cloth in the basket-rack assembly is replaced with a 40-mesh stainless steel cloth; a 10-mesh stainless-steel cloth is fitted to the top of the basket-rack assembly.
Time:
60 minutes.
Standard solution—
Transfer about 10 mg of USP Isotretinoin RS, accurately weighed, to a 200-mL low-actinic volumetric flask; add 25.0 mL of Stage 1 Medium and about 150 mL of Stage 2 Medium; sonicate until completely dissolved (about 20 minutes); and dilute with Stage 2 Medium to volume. Pass 20 mL of this solution through a suitable filter, discarding the first 5 mL. Dilute 5.0 mL of the filtrate with Stage 2 Medium to 50 mL.
Sample solution—
Perform a dissolution test on each of 6 Capsules: place 1 Capsule in one of the tubes in each of six basket-rack assemblies. Place each basket in a 1-L beaker containing 100 mL of Stage 1 Medium in a bath having a temperature of 37.0 ± 0.5 . Allow to stand for 30 minutes. Carefully add 800 mL of Stage 2 Medium to each beaker. With the disintegration apparatus operating, connect each basket-rack assembly to the drive rod in a timed sequence. After 60 minutes, withdraw 20 mL of Medium (Stage 1 and Stage 2), immediately pass the solution through a suitable 0.45-µm filter, discard the first 5 mL, and collect the solution in argon-charged, low-actinic glassware. Dilute, if necessary, using low-actinic glassware, with Stage 2 Medium, to obtain a theoretical concentration of about 0.0055 mg per mL of isotretinoin, assuming complete dissolution, based on the label claim.
. Allow to stand for 30 minutes. Carefully add 800 mL of Stage 2 Medium to each beaker. With the disintegration apparatus operating, connect each basket-rack assembly to the drive rod in a timed sequence. After 60 minutes, withdraw 20 mL of Medium (Stage 1 and Stage 2), immediately pass the solution through a suitable 0.45-µm filter, discard the first 5 mL, and collect the solution in argon-charged, low-actinic glassware. Dilute, if necessary, using low-actinic glassware, with Stage 2 Medium, to obtain a theoretical concentration of about 0.0055 mg per mL of isotretinoin, assuming complete dissolution, based on the label claim.
Capsule shell correction—
Empty the contents of 3 Capsules. Wash the Capsule shells in several 20-mL aliquots of chloroform. Allow the Capsule shells to air dry. Place the Capsule shells in a 1-L flask containing 100 mL of Stage 1 Medium and 800 mL of Stage 2 Medium. Allow the flask to stand for about 1 hour in a bath having a temperature of 37.0 ± 0.5 , stirring occasionally. Filter, and dilute as described for Sample solution.
, stirring occasionally. Filter, and dilute as described for Sample solution.
Procedure—
Determine the amount of C20H28O2 dissolved by employing UV absorption at the wavelength of maximum absorbance at about 343 nm, in portions of the Sample solution in comparison with the Standard solution, correcting for the Capsule shell absorbance, and using Medium (Stage 1 and Stage 2) as the blank. Calculate the percentage of C20H28O2 dissolved by the formula:
in which AU, ACS, and AS are the absorbances obtained from the Sample solution, the Capsule shell correction, and the Standard solution, respectively; CS is the concentration, in mg per mL, of the Standard solution; DU is the dilution factor of the Sample solution; 100 is the conversion factor to percentage; and LC is the Capsule label claim, in mg.
Tolerances—
Not less than 80% (Q) of the labeled amount of C20H28O2 is dissolved in 60 minutes.
test 2—
If the product complies with this test, the labeling indicates that the product meets USP Dissolution Test 2.
Medium:
0.05 M phosphate buffer, pH 7.8, containing 0.5% w/v solid N,N-dimethyldodecylamine N-oxide; 900 mL.
Apparatus 1:
20-mesh basket; 100 rpm.
Time:
90 minutes.
Buffer solution—
Transfer 3.4 g of monobasic potassium phosphate to a 1-L volumetric flask, dissolve in and dilute with water to volume, and adjust with phosphoric acid to a pH of 2.10 ± 0.05.
Mobile phase—
Prepare a filtered and degassed mixture of methanol and Buffer solution (81:19). Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard solution—
Transfer about 44 mg, accurately weighed, of USP Isotretinoin RS to a 100-mL low-actinic volumetric flask. Add 15 mL of 1-propanol, and sonicate for about 15 minutes. Add 50 mL of Medium, and sonicate for 10 minutes. Fill with Medium to volume. Transfer 5.0 mL to a 100-mL low-actinic volumetric flask, and dilute with Medium to volume. Dilute this solution with Medium to obtain a final concentration of about L/1000 mg/mL, where L is the Capsule label claim, in mg.
Test solution—
Pass a portion of the solution under test through a suitable 0.45-µm filter.
Chromatographic system—
The liquid chromatograph is equipped with a 358-nm detector and a 4.6-mm × 5-cm column containing 5-µm packing L1. The flow rate is about 2.0 mL per minute. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the tailing factor is not more than 2.0; the column efficiency is not less than 1000 theoretical plates; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 10 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the peak responses. Calculate the percentage of isotretinoin dissolved by the formula:
in which rU and rS are the peak responses for the Test solution and the Standard solution, respectively; CS is the concentration, in mg per mL, of the Standard solution; 900 is the volume, in mL, of Medium; 100 is the percentage conversion factor; and L is the Capsule label claim, in mg.
Tolerances—
Not less than 80% (Q) of the labeled amount of C20H28O2 is dissolved in 90 minutes.
test 3—
If the product complies with this test, the labeling indicates that the product meets USP Dissolution Test 3.
Medium:
borate buffer, pH 8.0, containing 0.5% cetrimide and 50 mg per L of pancreatin (Prepared by dissolving 12.37 g of boric acid and 14.91 g of potassium chloride in water and diluting with water to 1 L. To 250 mL of this solution, add 19.5 mL of 0.2 M sodium hydroxide solution, and dilute with water to 1 L. Adjust with 0.2 M sodium hydroxide to a pH of 8.00 ± 0.05, if necessary. Add 5 g of cetrimide, and mix until dissolved. Just before starting the test, dissolve a quantity of pancreatin to obtain a final concentration of 50 mg per L.); 900 mL.
Apparatus 2:
75 rpm, with sinkers.
Time:
90 minutes.
Standard solution—
Transfer about 45 mg, accurately weighed, of USP Isotretinoin RS to a 100-mL volumetric flask. Add 60 mL of 0.1 N sodium hydroxide, and sonicate until dissolved. Dilute with 0.1 N sodium hydroxide to volume. Dilute this solution with Medium to obtain a final concentration of about L/1000 mg/mL, where L is the Capsule label claim, in mg.
Test solution—
Pass a portion of the solution under test through a suitable 0.45-µm filter.
Mobile phase—
Prepare a filtered and degassed mixture of 0.5% acetic acid in methanol and 0.5% acetic acid in water (71:29). Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Chromatographic system—
The liquid chromatograph is equipped with a 353-nm detector and a 4.6-mm × 25-cm column containing 10-µm packing L1. The flow rate is about 1.5 mL per minute. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the tailing factor is not more than 2.0; the column efficiency is not less than 1800 theoretical plates; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 20 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the peak responses. Calculate the percentage of isotretinoin dissolved by the formula:
in rU and rS are the peak responses for the Test solution and the Standard solution, respectively; CS is the concentration, in mg per mL, of the Standard solution; 900 is the volume, in mL, of Medium; 100 is the percentage conversion factor; and L is the Capsule label claim, in mg.
Tolerances—
Not less than 70% (Q) of the labeled amount of C20H28O2 is dissolved in 90 minutes.
Uniformity of dosage units  905
905 :
meet the requirements.
:
meet the requirements.
Chromatographic purity—
Methylene chloride reagent—
Transfer 50 g of sodium bicarbonate to 1000 mL of methylene chloride, shake, and allow to stand overnight. At the time of use, filter suitable portions of this solution, and add 10 mg of butylated hydroxytoluene per mL.
Mobile phase—
Prepare a filtered and degassed mixture of hexanes, ethyl acetate, and glacial acetic acid (970:30:0.1). Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
System suitability solution—
Dissolve accurately weighed quantities of USP Isotretinoin RS and USP Tretinoin RS in Methylene chloride reagent to obtain a solution having known concentrations of about 1 mg of each Reference Standard per mL. Transfer 1.0 mL of this solution, and dilute quantitatively with hexanes to 100.0 mL to obtain a solution having known concentrations of about 0.01 mg of each Reference Standard per mL.
Standard solution—
Dissolve an accurately weighed quantity of USP Tretinoin RS in Methylene chloride reagent to obtain a solution having a known concentration of about 0.5 mg per mL. Dilute an accurately measured volume of this solution quantitatively, and stepwise if necessary, with hexanes to obtain a solution having a known concentration of about 1 µg per mL.
Test solution—
Weigh a number of Capsules, equivalent to about 200 mg of isotretinoin. With a sharp blade, carefully open the Capsules, without loss of material, and transfer the contents by pipetting 5 mL of Methylene chloride reagent over each Capsule and rinsing with hexanes. Collect the washings in a 500-mL volumetric flask, dilute with hexanes to volume, and mix. Transfer 50.0 mL of this solution to a 200-mL volumetric flask, dilute with hexanes to volume, and mix to obtain a solution having a concentration of about 0.1 mg of isotretinoin per mL.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 365-nm detector and a 4.6-mm × 25-cm column containing packing L3. The flow rate is about 1 mL per minute. Chromatograph the System suitability solution [note—The injection volume is about 20 µL. ] , and record the peak responses as directed for Procedure: the relative retention times for isotretinoin and tretinoin are about 0.75 and 1.00, respectively; the resolution, R, between isotretinoin and tretinoin is not less than 3.0; the tailing factor for the isotretinoin peak is not greater than 2.5; and the relative standard deviation for replicate injections is not more than 2.0%.
)—
The liquid chromatograph is equipped with a 365-nm detector and a 4.6-mm × 25-cm column containing packing L3. The flow rate is about 1 mL per minute. Chromatograph the System suitability solution [note—The injection volume is about 20 µL. ] , and record the peak responses as directed for Procedure: the relative retention times for isotretinoin and tretinoin are about 0.75 and 1.00, respectively; the resolution, R, between isotretinoin and tretinoin is not less than 3.0; the tailing factor for the isotretinoin peak is not greater than 2.5; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 50 µL) of the Standard solution and the Test solution into the chromatograph, and allow the Test solution to elute for not less than two times the retention time of isotretinoin. Record the chromatograms, and measure the peak responses: the peak response for any impurity is not more than that of the tretinoin response obtained from the Standard solution (1.0%); and the sum of all the peak responses, excluding that of isotretinoin, obtained from the Test solution, is not more than 1.5 times the tretinoin response obtained from the Standard solution (1.5%).
Assay—
[note—Protect the System suitability solution, the Standard preparation, the Assay stock preparation, and the Assay preparation from direct light. ]
Diluent—
Heat 0.1 N sodium hydroxide to about 60 to 70
to 70 . Cool it to room temperature and purge with helium or nitrogen. Store it in a plastic container.
. Cool it to room temperature and purge with helium or nitrogen. Store it in a plastic container.
Solvent A—
Prepare a solution of 0.5% acetic acid in methanol.
Solvent B—
Prepare a solution of 0.5% acetic acid in water.
Mobile phase—
Prepare a mixture of Solvent A and Solvent B (71:29). Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
System suitability solution—
Dissolve suitable quantities of USP Isotretinoin RS and USP Tretinoin RS in Diluent to obtain a solution having known concentrations of about 0.04 mg per mL of isotretinoin and 0.02 mg per mL of tretinoin.
Standard preparation—
Dissolve an accurately weighed quantity of USP Isotretinoin RS in Diluent, and dilute with Diluent, stepwise if necessary, to obtain a solution having a known concentration of about 0.04 mg per mL.
Assay stock preparation—
Transfer not fewer than 10 Capsules to a suitable volumetric flask. Add Diluent to the volumetric flask to fill about 50% of the volume, sonicate for 1 hour with occasional shaking to disperse all the contents, and make up the volume with Diluent to obtain a solution having a known concentration of about 0.4 mg per mL of isotretinoin.
Assay preparation—
Transfer 5 mL of the Assay stock preparation to a 50-mL volumetric flask, and dilute with Diluent to volume. Pass the solution through a suitable 0.45-µm or finer porosity membrane filter.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 353-nm detector and a 4.6-mm × 25-cm column containing packing L1. The flow rate is about 1.5 mL per minute. Chromatograph the System suitability solution, and record the peak areas as directed for Procedure: the relative retention times for isotretinoin and tretinoin are about 1.0 and 1.3, respectively; the resolution, R, between isotretinoin and tretinoin is not less than 2.0; the tailing factor for the isotretinoin peak is not greater than 2.0; and the column efficiency determined from the isotretinoin peak is not less than 2000. Chromatograph the Standard preparation, and record the peak areas as directed for Procedure: the relative standard deviation for replicate injections is not more than 2.0%.
)—
The liquid chromatograph is equipped with a 353-nm detector and a 4.6-mm × 25-cm column containing packing L1. The flow rate is about 1.5 mL per minute. Chromatograph the System suitability solution, and record the peak areas as directed for Procedure: the relative retention times for isotretinoin and tretinoin are about 1.0 and 1.3, respectively; the resolution, R, between isotretinoin and tretinoin is not less than 2.0; the tailing factor for the isotretinoin peak is not greater than 2.0; and the column efficiency determined from the isotretinoin peak is not less than 2000. Chromatograph the Standard preparation, and record the peak areas as directed for Procedure: the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 20 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the areas for the major peaks. Calculate the quantity, in mg, of isotretinoin (C20H28O2) in each of the Capsules taken by the formula:
10(CV/N)(rU / rS)
in which C is the concentration, in mg per mL, of isotretinoin in the Standard preparation; V is the volume, in mL, of the volumetric flask used to prepare the Assay stock preparation; N is the number of Capsules taken; and rU and rS are the isotretinoin peak areas obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Feiwen Mao, M.S.
Senior Scientific Liaison 1-301-816-8320 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
|
| Margareth R.C. Marques, Ph.D.
Senior Scientific Liaison 1-301-816-8106 |
(GCDF2010) General Chapters - Dosage Forms |
USP35–NF30 Page 3598
Pharmacopeial Forum: Volume No. 34(4) Page 942