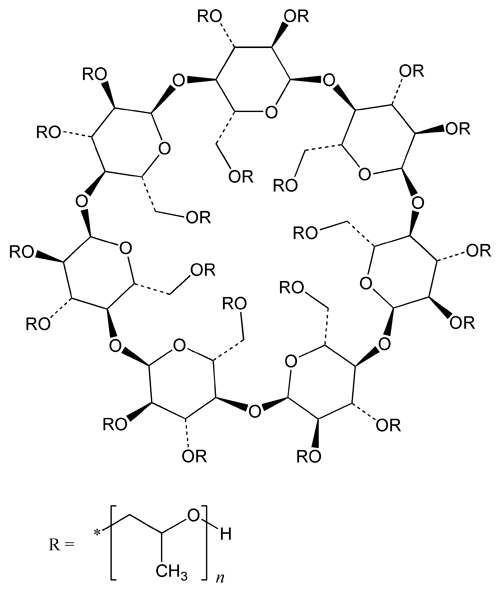
Hydroxypropyl Betadex
(hye drox'' ee proe' pil bay' ta dex).
Beta cyclodextrin, 2-hydroxypropyl ether
» Hydroxypropyl Betadex is a partially substituted poly(hydroxypropyl) ether of Betadex. The number of hydroxypropyl groups per anhydroglucose unit expressed as molar substitution (MS) is not less than 0.40 and not more than 1.50 and is within 10 percent of the value stated on the label.
Packaging and storage—
Preserve in well-closed containers. Store at room temperature.
Labeling—
Label it to indicate the molar substitution (MS). Where Hydroxypropyl Betadex is intended for use in the manufacture of injectable dosage forms, it is so labeled. Where Hydroxypropyl Betadex must be subjected to further processing during the preparation of injectable dosage forms to ensure acceptable levels of bacterial endotoxins, it is so labeled. Where Hydropropyl Betadex is sterile, it is so labeled.
USP Reference standards  11
11 —
—
USP Endotoxin RS
Clarity of solution—
Dissolve 1.0 g in 2.0 mL of water, and heat: the resulting solution is clear and remains transparent after cooling to room temperature.
Identification—
A:
Infrared Absorption  197K
197K —The spectrum obtained with Hydroxypropyl Betadex shows the same absorption bands as the spectrum acquired with USP Hydroxypropyl Betadex RS. Due to the difference in the substitution of the substance, the intensity of some absorption bands may vary.
—The spectrum obtained with Hydroxypropyl Betadex shows the same absorption bands as the spectrum acquired with USP Hydroxypropyl Betadex RS. Due to the difference in the substitution of the substance, the intensity of some absorption bands may vary.
B:
It meets the requirements of the test for Clarity of solution.
Microbial enumeration tests  61
61 and Tests for specified microorganisms
and Tests for specified microorganisms  62
62 —
The total aerobic microbial count does not exceed 1000 cfu per g, and the total combined molds and yeasts count does not exceed 100 cfu per g.
—
The total aerobic microbial count does not exceed 1000 cfu per g, and the total combined molds and yeasts count does not exceed 100 cfu per g.
Heavy metals, Method I  231
231 :
not more than 20 µg per g.
:
not more than 20 µg per g.
Loss on drying  731
731 —
Dry about 1 g of it at 120
—
Dry about 1 g of it at 120 for 2 hours: it loses not more than 10.0% of its weight.
for 2 hours: it loses not more than 10.0% of its weight.
Conductivity—
Test solution—
Transfer about 5.0 g of Hydroxypropyl Betadex, accurately weighed and calculated on the dried basis, to a 50-mL volumetric flask, dissolve in and dilute with water (previously boiled and cooled to room temperature) to volume, and mix.
Apparatus—
Use a conductivity meter or resistivity meter that measures the resistance of the column of liquid between the electrodes of the immersed measuring device. The apparatus is supplied with alternating current to avoid the effects of electrode polarization. It is equipped with a temperature compensation device or a precision thermometer.
Reagents—
Prepare three standard solutions of potassium chloride containing 0.7455, 0.0746, and 0.0149 g, respectively, of potassium chloride per 1000.0 g of solution. These solutions should be prepared using water, which has been previously boiled and cooled to room temperature and whose conductivity does not exceed 2 µS·cm–1. The conductivity and resistivity of these three solutions at 20 are given below.
are given below.
| Concentration of solution in g/1000.0 g |
Conductivity µS·cm–1 |
Resistivity |
|---|---|---|
| 0.7455 | 1330 | 752 |
| 0.0746 | 133.0 | 7519 |
| 0.0149 | 26.6 | 37,594 |
Calibration—
Choose a conductivity cell that is appropriate for the conductivity of the solution to be examined. The higher the expected conductivity, the higher the cell constant that must be chosen. Commonly used conductivity cells have cell constants of the order of 0.1 cm–1, 1 cm–1, and 10 cm–1. Use a Standard solution of potassium chloride that is appropriate for the measurement. The conductivity value of the Standard solution of potassium chloride should be near the expected conductivity value of the Test solution. Rinse the cell several times with water, which has been previously boiled and cooled to room temperature, and at least twice with the potassium chloride solution used for the determination of the cell constant of the conductivity cell. Measure the resistance of the conductivity cell using the potassium chloride solution at 20 ± 0.1 . The constant C (in cm–1) of the conductivity cell is given by the expression:
. The constant C (in cm–1) of the conductivity cell is given by the expression:
C = RKCl × KKCl
in which RKCl is the measured resistance, expressed in mega-ohms; and KKCl is the conductivity of the standard solution of potassium chloride used, expressed in µS·cm–1. The measured constant, C, of the conductivity cell must be within 5% of the given value.
Procedure—
Rinse the conductivity cell several times with water, which has been previously boiled and cooled to room temperature, and at least twice with the Test solution. Measure the conductivity of the Test solution, while gently stirring with a magnetic stirrer: the conductivity is not more than 200 µS·cm–1.
Bacterial endotoxins  85
85 —
The level of bacterial endotoxins is such that the requirement under the relevant dosage form monograph(s) in which Hydropropyl Betadex is used can be met. Where the label states that Hydroxypropyl Betadex must be subjected to further processing during the preparation of injectable dosage forms, the level of bacterial endotoxins is such that the requirement under the relevant dosage form monograph(s) in which Hydroxypropyl Betadex is used can be met.
—
The level of bacterial endotoxins is such that the requirement under the relevant dosage form monograph(s) in which Hydropropyl Betadex is used can be met. Where the label states that Hydroxypropyl Betadex must be subjected to further processing during the preparation of injectable dosage forms, the level of bacterial endotoxins is such that the requirement under the relevant dosage form monograph(s) in which Hydroxypropyl Betadex is used can be met.
Sterility  71
71 —
Where the label states that Hydropropyl Betadex is sterile, it meets the requirements for Sterility
—
Where the label states that Hydropropyl Betadex is sterile, it meets the requirements for Sterility  71
71 in the relevant dosage form monograph(s) in which Hydropropyl Betadex is used.
in the relevant dosage form monograph(s) in which Hydropropyl Betadex is used.
Related compounds—
Mobile phase—
Use water.
Standard solution A—
Dissolve accurately weighed quantities of USP Beta Cyclodextrin RS and USP Propylene Glycol RS in water to make a solution having a known concentration of about 15 mg per mL for beta cyclodextrin, calculated on the anhydrous basis, and about 25 mg per mL for propylene glycol.
Standard solution B—
Pipet 1.0 mL of Standard solution A into a 10-mL volumetric flask, dilute with water to volume, and mix.
Test solution—
Dissolve 2.50 g of Hydroxypropyl Betadex, accurately weighed and calculated on the dried basis, in water with the aid of heat. Cool, and dilute with water to 25.0 mL.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a differential refractometer detector and a 3.9-mm × 30-cm column and pre-column that contain packing L11, both maintained at a temperature of 40
)—
The liquid chromatograph is equipped with a differential refractometer detector and a 3.9-mm × 30-cm column and pre-column that contain packing L11, both maintained at a temperature of 40 . The flow rate is about 1.5 mL per minute. Chromatograph Standard solution A and Standard solution B, and record the peak responses as directed for Procedure: the resolution, R, between betadex and propylene glycol is not less than 4 for Standard solution A; and the relative standard deviation for replicate injections of Standard solution B is not more than 2.0%. [note—For information purposes only, the retention time of propylene glycol is about 2.5 minutes, and the relative retention times with reference to that of propylene glycol are about 4.2 for betadex and about 6 for hydroxypropyl betadex; Hydroxypropyl Betadex elutes as a very wide peak or several peaks. ]
. The flow rate is about 1.5 mL per minute. Chromatograph Standard solution A and Standard solution B, and record the peak responses as directed for Procedure: the resolution, R, between betadex and propylene glycol is not less than 4 for Standard solution A; and the relative standard deviation for replicate injections of Standard solution B is not more than 2.0%. [note—For information purposes only, the retention time of propylene glycol is about 2.5 minutes, and the relative retention times with reference to that of propylene glycol are about 4.2 for betadex and about 6 for hydroxypropyl betadex; Hydroxypropyl Betadex elutes as a very wide peak or several peaks. ]
Procedure—
Separately inject equal volumes (about 20 µL) of Standard solution B and the Test solution into the chromatograph, record the chromatograms, and measure the responses for the major peaks, disregarding any peaks eluting before propylene glycol and after the hydroxypropyl betadex peak. The area of the betadex peak in the Test solution is not more than the area of the corresponding peak in the chromatogram obtained with Standard solution B (1.5%). The area of the propylene glycol peak in the Test solution is not more than the area of the corresponding peak in the chromatogram obtained with Standard solution B (2.5%). The area obtained from any other single impurity peak is not more than 0.1 times the area of propylene glycol in the chromatogram obtained with Standard solution B (0.25%). The total area obtained from all impurity peaks, excluding betadex and propylene glycol, is not more than 0.4 times the area of propylene glycol in the chromatogram obtained with Standard solution B (1%). Disregard any peaks that are less than 0.04 times the area of propylene glycol in the chromatogram obtained with Standard solution B (0.1%).
Molar substitution (see Nuclear Magnetic Resonance  761
761 )—
The molar substitution (MS) is calculated from the ratio between the signal from the three protons of the methyl group, contained in the hydroxypropyl functional group, and the signal from the proton attached to the carbon C1 (glycosidic proton) of the anhydroglucose units. Use a Fourier-transform nuclear magnetic resonance (NMR) spectrometer having a magnetic field strength of at least 6 Tesla and that is capable of performing quantitative analysis using proton NMR spectroscopy at a temperature of at least 25
)—
The molar substitution (MS) is calculated from the ratio between the signal from the three protons of the methyl group, contained in the hydroxypropyl functional group, and the signal from the proton attached to the carbon C1 (glycosidic proton) of the anhydroglucose units. Use a Fourier-transform nuclear magnetic resonance (NMR) spectrometer having a magnetic field strength of at least 6 Tesla and that is capable of performing quantitative analysis using proton NMR spectroscopy at a temperature of at least 25 .
.
Test preparation—
Mix not less than the equivalent of 10.0 mg of dried Hydroxypropyl Betadex with 0.75 mL of deuterium oxide thoroughly in an NMR tube. Place the tube into an NMR probe.
Procedure—
Adjust the spectrometer settings so that a high-resolution proton NMR spectrum can be acquired that will provide quantitative data. Acquire a free induction decay (FID) with at least 8 transients using a spectral window from at least 0 to 6.2 ppm, with the solvent peak located at 4.8 ppm at 25 . Zero fill the spectrum at least 3 times, and Fourier transform the FID with no Gaussian line broadening and no more than 0.2 Hz of Lorenzian line broadening. Determine the peak areas of the doublet from the methyl protons of the hydroxypropyl functional group at 1.2 ppm (A1) and the peak areas from the glycosidic protons, which are located between 5 and 5.4 ppm (A2). Calculate the molar substitution by the formula:
. Zero fill the spectrum at least 3 times, and Fourier transform the FID with no Gaussian line broadening and no more than 0.2 Hz of Lorenzian line broadening. Determine the peak areas of the doublet from the methyl protons of the hydroxypropyl functional group at 1.2 ppm (A1) and the peak areas from the glycosidic protons, which are located between 5 and 5.4 ppm (A2). Calculate the molar substitution by the formula:
A1/(3A2)
in which A1 is the area of the methyl group of hydroxypropyl; and A2 is the area of the glycosidic proton. The degree of substitution is the number of hydroxypropyl groups per molecule of betadex and is obtained by multiplying the MS by 7.
Limit of propylene oxide—
Ether stock solution—
Add 75 µL of ether to about 30 mL of dimethylacetamide in a 50-mL volumetric flask, dilute with dimethylacetamide to volume, and mix. This solution contains about 1.0 mg per mL of ether.
Internal standard solution—
Add 30 µL of Ether stock solution to about 70 mL of dimethylacetamide in a 100-mL volumetric flask, dilute with dimethylacetamide to volume, and mix.
Propylene oxide stock solution—
[Caution—Propylene oxide is toxic and flammable. Prepare this solution in a well-ventilated fume hood.
] Add about 30 mL of dimethylacetamide into a 50-mL volumetric flask. Weigh the flask and contents accurately, add 60 µL of propylene oxide (cooled in a refrigerator) into the flask with a 100-µL cooled microsyringe, weigh again, and calculate the weight of propylene oxide added, by difference. [note—Propylene oxide is a gas at room temperature. It is usually stored in a lecture-type gas cylinder or small metal pressure bomb. Chill the cylinder in a refrigerator before use. Transfer about 5 mL of the liquid propylene oxide to a 100-mL beaker chilled in wet ice. Use a gas-tight syringe that has been chilled in a refrigerator. ] Dilute with dimethylacetamide to volume, and mix. This solution contains about 1.0 mg per mL of propylene oxide.
Resolution solution—
Add 30 µL of the Ether stock solution and 20 µL of Propylene oxide stock solution to about 70 mL of dimethylacetamide in a 100-mL volumetric flask, dilute with dimethylacetamide to volume, and mix.
Standard stock solutions—
Add about 7 mL of dimethylacetamide into each of four 10-mL volumetric flasks. Transfer the following amount of Propylene oxide stock solution into each of the four flasks using a microsyringe, with one amount per flask: 40, 100, 200, and 400 µL. Dilute with dimethylacetamide to volume, and mix. The Standard stock solutions contain about 4, 10, 20, and 40 µg per mL of propylene oxide, respectively.
Standard solutions—
Into each of four 10-mL headspace vials, transfer 200 ± 5 mg of Hydroxypropyl Betadex, calculated on the dried basis. Pipet 1.0 mL of the Internal standard solution into each vial, and close the vial with septum and cap. Into each of the vials, add 10 µL of each of Standard stock solutions using a 10-µL syringe, respectively. Allow each vial to stand, and gently shake until the sample is dissolved. The Standard solutions contain, respectively, about 0.04, 0.1, 0.2, and 0.4 µg per mL of propylene oxide.
Test solution—
Transfer 200 ± 5 mg of Hydroxypropyl Betadex, calculated on the dried basis, into a 10-mL headspace autosampler vial. Pipet 1.0 mL of the Internal standard solution into the vial, and close the vial with a septum and cap. Add 10 µL of dimethylacetamide using a 10-µL syringe. Allow the vial to stand, and gently shake until the sample is dissolved.
Chromatographic system (see Chromatography  621
621 )—
The gas chromatograph is equipped with a balanced pressure automatic headspace sampler with a split injection mode of a ratio of 1:1, a flame-ionization detector and a 0.32-mm × 10-m fused-silica capillary column coated with a 10-µm layer of stationary phase S3. The column temperature is maintained at 50
)—
The gas chromatograph is equipped with a balanced pressure automatic headspace sampler with a split injection mode of a ratio of 1:1, a flame-ionization detector and a 0.32-mm × 10-m fused-silica capillary column coated with a 10-µm layer of stationary phase S3. The column temperature is maintained at 50 for the first 10 minutes after injection, programmed to rise at a rate of 10
for the first 10 minutes after injection, programmed to rise at a rate of 10 per minute to a temperature of 100
per minute to a temperature of 100 , maintained for 10 minutes at 100
, maintained for 10 minutes at 100 , then is increased at a rate of 20
, then is increased at a rate of 20 per minute to a temperature of 220
per minute to a temperature of 220 , and maintained at 220
, and maintained at 220 for 4 minutes. The transfer line temperature is maintained at 120
for 4 minutes. The transfer line temperature is maintained at 120 . The detector temperature is maintained at 250
. The detector temperature is maintained at 250 and the injection port temperature is maintained at 120
and the injection port temperature is maintained at 120 . The carrier gas is helium, flowing at a rate of about 2.0 mL per minute, corresponding to the linear velocity of 44 cm per second. Chromatograph the Resolution solution, and record the peak response as directed for Procedure: the resolution, R, between ether and propylene oxide is not less than 2.0. [note—For information purposes only, the relative retention times are about 1.0 for propylene oxide and 1.3 for ether. ]
. The carrier gas is helium, flowing at a rate of about 2.0 mL per minute, corresponding to the linear velocity of 44 cm per second. Chromatograph the Resolution solution, and record the peak response as directed for Procedure: the resolution, R, between ether and propylene oxide is not less than 2.0. [note—For information purposes only, the relative retention times are about 1.0 for propylene oxide and 1.3 for ether. ]
Procedure—
Separately place the vials containing the Standard solutions and the Test solution in the automated sampler, and start the sequence so that the vial is heated at a temperature of 100 for 30 minutes before a suitable portion of its headspace is injected into the chromatograph. Using a 2-mL gas syringe preheated in an oven at 110
for 30 minutes before a suitable portion of its headspace is injected into the chromatograph. Using a 2-mL gas syringe preheated in an oven at 110 , separately inject 1.0 mL of the headspace from each vial into the chromatograph. Chromatograph the Standard solutions and the Test solution, record the chromatograms, and measure the area ratios of the peak responses of propylene oxide and ether as directed for Procedure. Determine, based on a retention time comparison, whether propylene oxide is detected in the Test solution. Plot the area ratios of the peak responses of propylene oxide and ether of the Test solution and the Standard solutions versus the content, in µg, of propylene oxide in each vial, as furnished by the Standard stock solutions, draw the straight line best fitting the five points, and calculate the correlation coefficient for the line. [note—The Test solution should be plotted as if it had a content of added propylene oxide equivalent to 0 µg. ] A suitable system is one that yields a line having a correlation coefficient of not less than 0.99. Extrapolate the line until it meets the content axis on the negative side. The distance between this point and the intersection of the axes represents the total amount, TU, in µg, of propylene oxide in the Test solution. Calculate the percentage of propylene oxide in the portion of Hydroxypropyl Betadex taken by the formula:
, separately inject 1.0 mL of the headspace from each vial into the chromatograph. Chromatograph the Standard solutions and the Test solution, record the chromatograms, and measure the area ratios of the peak responses of propylene oxide and ether as directed for Procedure. Determine, based on a retention time comparison, whether propylene oxide is detected in the Test solution. Plot the area ratios of the peak responses of propylene oxide and ether of the Test solution and the Standard solutions versus the content, in µg, of propylene oxide in each vial, as furnished by the Standard stock solutions, draw the straight line best fitting the five points, and calculate the correlation coefficient for the line. [note—The Test solution should be plotted as if it had a content of added propylene oxide equivalent to 0 µg. ] A suitable system is one that yields a line having a correlation coefficient of not less than 0.99. Extrapolate the line until it meets the content axis on the negative side. The distance between this point and the intersection of the axes represents the total amount, TU, in µg, of propylene oxide in the Test solution. Calculate the percentage of propylene oxide in the portion of Hydroxypropyl Betadex taken by the formula:
100(TU /W)
in which W is the weight, in µg, of Hydroxypropyl Betadex taken to prepare the Test solution: the limit is 0.0001%.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Robert H. Lafaver, M.S.
Scientific Liaison 1-301-816-8335 |
(EXC2010) Monographs - Excipients |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
|
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology |
USP35–NF30 Page 1819
Pharmacopeial Forum: Volume No. 32(5) Page 1481