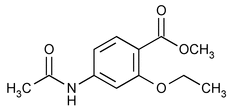
Ethopabate
(eth'' oh pab' ate).
Benzoic acid, 4-(acetylamino)-2-ethoxy-, methyl ester.
Methyl 4-acetamido-2-ethoxybenzoate
» Ethopabate contains not less than 96.0 percent and not more than 101.0 percent of C12H15NO4, calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers, protected from light.
Labeling—
Label it to indicate that it is for veterinary use only.
Identification—
Solution:
10 µg per mL.
Medium:
methanol.
Loss on drying  731
731 —
Dry 1.0 g of it in vacuum at 60
—
Dry 1.0 g of it in vacuum at 60 for 2 hours: it loses not more than 1.0% of its weight.
for 2 hours: it loses not more than 1.0% of its weight.
Melting range  741
741 :
between 146
:
between 146 and 151
and 151 .
.
Residue on ignition  281
281 :
not more than 0.5%.
:
not more than 0.5%.
Chromatographic purity—
Examine the chromatogram of the Assay preparation, as obtained in the Assay, for peaks that elute at the following retention times in relation to ethopabate: 0.33, p-aminosalicylic acid (ethopabate related compound B); 0.64, methyl 2-ethoxy-4-aminobenzoate (ethopabate related compound C); 0.68, methyl 2-hydroxy-4-aminobenzoate (ethopabate related compound D); 0.9, methyl 4-acetamido-2-hydroxybenzoate (ethopabate related compound A); and 1.6, ethyl 4-acetamido-2-ethoxybenzoate (ethopabate related compound E). Calculate the percentage of diazotizable substances, represented by peaks for ethopabate related compounds B, C, and D, if present, by the formula:
(0.72rB + 0.68rC + 0.74rD) / 0.01rU
in which 0.72, 0.68, and 0.74 are the response factors of ethopabate related compounds B, C, and D, respectively, relative to that of ethopabate, rB, rC, and rD are the responses of the peaks observed for ethopabate related compounds B, C, and D, respectively, and rU is the ethopabate peak response obtained from the Assay preparation: not more than 0.5% of diazotizable substances is found. Calculate the percentage of any other impurities by the formula:
100  AE
AE  As
As
in which AE is the percentage of total peak area represented by the main ethopabate peak in the chromatogram obtained from the Assay preparation, and As is the percentage of peak area represented by the sum of the peaks for ethopabate related compounds B, C, and D: not more than 2.0% of other impurities is found. [note—Exclude from the total peak area the responses of any minor peaks that are 0.01% or less than that of the main ethopabate peak. ]
Assay—
Mobile phase—
Dissolve 3 g of sodium 1-hexanesulfonate in 1 L of water, and adjust with phosphoric acid to a pH of 2.5. Prepare a filtered and degassed mixture of this solution, methanol, and acetonitrile (450:150:30). Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Diluent—
Prepare a mixture of methanol and water (50:50).
Standard preparation—
Prepare a solution of USP Ethopabate RS in Diluent having a known concentration of about 0.4 mg per mL. If necessary, filter this solution through a filter having a porosity of 0.5 µm or finer, and use the filtrate as the Standard preparation. Use this solution on the day prepared.
Assay preparation—
Transfer about 40 mg of Ethopabate, accurately weighed, to a 100-mL volumetric flask, add about 80 mL of Diluent, and dissolve with the aid of sonication. Mix and dilute to volume with Mobile phase. If necessary, filter this solution through a filter having a porosity of 0.5 µm or finer, and use the filtrate as the Assay preparation. Use this solution on the day prepared.
Resolution solution—
Prepare a solution in Diluent containing about 0.4 mg of USP Ethopabate RS and 0.1 mg of USP Ethopabate Related Compound A RS per mL.
Chromatographic system
(see Chromatography  621
621 )—The liquid chromatograph is equipped with a 268-nm detector and a 3.9-mm × 30-cm column that contains packing L11 and is maintained at about 40
)—The liquid chromatograph is equipped with a 268-nm detector and a 3.9-mm × 30-cm column that contains packing L11 and is maintained at about 40 . The flow rate is about 1 mL per minute. Chromatograph the Resolution solution, and record the peak responses as directed under Procedure: the relative retention times are about 0.9 for methyl 4 acetamido-2-hydroxybenzoate (ethopabate related compound A) and 1.0 for ethopabate, the column efficiency is not less than 4000 theoretical plates, the resolution, R, between the ethopabate related compound A peak and the ethopabate peak is not less than 1.2, and the tailing factor is not more than 1.5. Chromatograph the Standard preparation, and record the peak responses as directed under Procedure: the relative standard deviation for replicate injections is not more than 1.0%.
. The flow rate is about 1 mL per minute. Chromatograph the Resolution solution, and record the peak responses as directed under Procedure: the relative retention times are about 0.9 for methyl 4 acetamido-2-hydroxybenzoate (ethopabate related compound A) and 1.0 for ethopabate, the column efficiency is not less than 4000 theoretical plates, the resolution, R, between the ethopabate related compound A peak and the ethopabate peak is not less than 1.2, and the tailing factor is not more than 1.5. Chromatograph the Standard preparation, and record the peak responses as directed under Procedure: the relative standard deviation for replicate injections is not more than 1.0%.
Procedure—
Separately inject equal volumes (about 10 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the areas of the responses for the major peaks. Calculate the quantity, in mg, of C12H15NO4 in the portion of Ethopabate taken by the formula:
100C(rU / rS)
in which C is the concentration, in mg per mL, of USP Ethopabate RS in the Standard preparation, and rU and rS are the ethopabate peak responses obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Morgan Puderbaugh, B.S.
Associate Scientific Liaison 1-301-998-6833 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3130