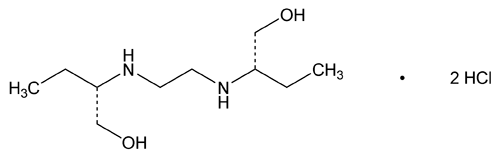
Ethambutol Hydrochloride
C10H24N2O2·2HCl 277.23
1-Butanol, 2,2¢-(1,2-ethanediyldiimino)bis-, dihydrochloride, [S-(R*,R*)]-;
(+)-2,2¢-(Ethylenediimino)-di-1-butanol dihydrochloride

 [1070-11-7].
[1070-11-7].
(eth am' bue tol hye'' droe klor' ide).
C10H24N2O2·2HCl 277.23
1-Butanol, 2,2¢-(1,2-ethanediyldiimino)bis-, dihydrochloride, [S-(R*,R*)]-;
(+)-2,2¢-(Ethylenediimino)-di-1-butanol dihydrochloride
DEFINITION
Ethambutol Hydrochloride contains NLT 98.0% and NMT 100.5% of C10H24N2O2·2HCl, calculated on the dried basis.
IDENTIFICATION
ASSAY
• Procedure
Sample solution:
200 mg of Ethambutol Hydrochloride in a mixture of 100 mL of glacial acetic acid and 5 mL of mercuric acetate TS. Add crystal violet TS.
Analysis:
Titrate with 0.1 N perchloric acid VS (the color change at the endpoint is from blue to blue-green). Perform a blank determination, and make any necessary corrections (see Titrimetry  541
541 ). Each mL of 0.1 N perchloric acid is equivalent to 13.86 mg of C10H24N2O2·2HCl.
). Each mL of 0.1 N perchloric acid is equivalent to 13.86 mg of C10H24N2O2·2HCl.
Acceptance criteria:
98.0%–100.5% on the dried basis
IMPURITIES
Inorganic Impurities
• Heavy Metals, Method II  231
231 :
NMT 20 ppm
:
NMT 20 ppm
Organic Impurities
• Procedure 1: Ordinary Impurities  466
466
Sample solution:
Methanol
Standard solution:
Methanol
Eluant:
Methanol and ammonium hydroxide (18:1)
Visualization:
16
• Procedure 2: Limit of Aminobutanol
Solution A:
1.24 g of boric acid to a 100-mL volumetric flask. Dissolve in 90 mL of water, adjust with 5 N sodium hydroxide to a pH of 9.0, and dilute with water to volume.
Fluorescamine solution:
0.1 mg/mL of fluorescamine in acetone
Standard solution:
5.0 µg/mL of USP Aminobutanol RS
Sample solution:
0.5 mg/mL of Ethambutol Hydrochloride
Spectrometric conditions
Mode:
Fluorometry
Analytical wavelength:
485 nm, with the excitation wavelength at about 385 nm
Cell:
1 cm
Analysis
Samples:
Standard solution and Sample solution
Pipet a 10-mL portion of the Sample solution into a glass-stoppered, 100-mL conical flask, and add 10 mL of water and 20 mL of Solution A. To another 100-mL flask, add 10.0 mL of the Sample solution, 10.0 mL of the Standard solution, and 20 mL of Solution A. Place the flasks on a magnetic stirrer, and while the contents are being stirred rapidly, add 10 mL of Fluorescamine solution rapidly. Insert the stoppers in the flasks, invert, and shake briefly. After 1 min, accurately timed, determine the relative fluorescence intensities of both solutions in a suitable fluorometer.
Acceptance criteria:
The fluorescence intensity of the solution from the Sample solution is NMT the difference between the intensities of the two solutions (NMT 1.0%).
SPECIFIC TESTS
• Limit of Total Stereoisomers
Solution A:
60 mg/mL of (R)-(+)-alpha-methylbenzyl isocyanate in acetonitrile
Solution B:
Acetonitrile and water (1:1)
Mobile phase:
Methanol and water (13:7)
Standard solution 1:
Transfer 13 mg of USP Ethambutol Hydrochloride RS to a 10-mL volumetric flask. Add 2.0 mL acetonitrile and 260 µL of triethylamine, and mix for 1 min. Add 650 µL of Solution A, and mix for another min. Dilute with Solution B to volume.
Standard solution 2:
Prepare as directed for Standard solution 1 using USP Ethambutol Related Compound A RS and USP Ethambutol Related Compound B RS.
System suitability solution:
Mix equal volumes of Standard solution 1 and Standard solution 2.
Sample solution:
Prepare as directed for Standard solution 1 using Ethambutol Hydrochloride.
Chromatographic system
Mode:
LC
Detector:
UV 254 nm
Column:
3.9-mm × 15-cm; packing L1
Flow rate:
1.7 mL/min
Injection size:
20 µL
Run time:
2.3 times the retention time of ethambutol
System suitability
Samples:
Standard solution 1 and System suitability solution
[Note—The relative retention times for ethambutol related compound B, ethambutol, and ethambutol related compound A are 0.85, 1.0, and 1.4, respectively. ]
Suitability requirements
Resolution:
NLT 3.0 between ethambutol and ethambutol related compound A, System suitability solution
Relative standard deviation:
NMT 2.0%, Standard solution 1
Analysis
Sample:
Sample solution
Calculate the percentage of stereoisomers in the portion of Sample taken:
Result = (rU/rT) × 100
| rU | = | = peak response of ethambutol related compound A or ethambutol related compound B from the Sample solution |
| rT | = | = sum of all peak responses of ethambutol related compound A, ethambutol related compound B and ethambutol from the Sample solution |
Calculate the percentage of total stereoisomers:
Result = % ethambutol related compound A + % ethambutol related compound B
Acceptance criteria
Total impurities:
NMT 4.0%
• Loss on Drying  731
731 :
Dry a sample at 105
:
Dry a sample at 105 for 2 h: it loses NMT 0.5% of its weight.
for 2 h: it loses NMT 0.5% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed containers.
• USP Reference Standards  11
11
USP Ethambutol Related Compound A RS
(2R,2¢S)-2,2¢-[Ethane-1,2-diylbis(azanediyl)]dibutan-1-ol.
C10H24N2O2 204.31
(2R,2¢S)-2,2¢-[Ethane-1,2-diylbis(azanediyl)]dibutan-1-ol.
C10H24N2O2 204.31
USP Ethambutol Related Compound B RS
(2R,2¢R)-2,2¢-[Ethane-1,2-diylbis(azanediyl)]dibutan-1-ol.
C10H24N2O2 204.31
(2R,2¢R)-2,2¢-[Ethane-1,2-diylbis(azanediyl)]dibutan-1-ol.
C10H24N2O2 204.31
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Behnam Davani, Ph.D., M.B.A.
Senior Scientific Liaison 1-301-816-8394 |
(SM12010) Monographs - Small Molecules 1 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3123
Pharmacopeial Forum: Volume No. 36(1) Page 98