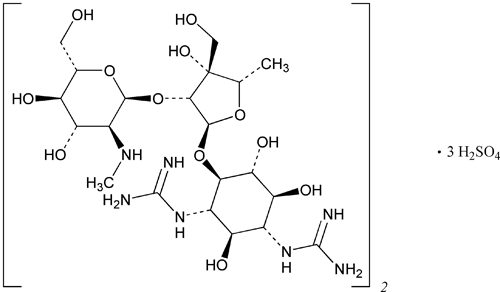
Dihydrostreptomycin Sulfate
(dye hye'' droe strep'' toe mye' sin sul' fate).
» Dihydrostreptomycin Sulfate has a potency equivalent to not less than 650 µg of dihydrostreptomycin (C21H41N7O12) per mg, except that if it is labeled as being crystalline, it has a potency equivalent to not less than 725 µg of dihydrostreptomycin per mg, or if it is labeled as being solely for oral use, it has a potency equivalent to not less than 450 µg of dihydrostreptomycin per mg.
Dihydrostreptomycin Sulfate has a potency equivalent to not less than 650 µg of dihydrostreptomycin (C21H41N7O12) per mg, except that if it is labeled as being crystalline, it has a potency equivalent to not less than 725 µg of dihydrostreptomycin per mg, or if it is labeled as being solely for oral use, it has a potency equivalent to not less than 450 µg of dihydrostreptomycin per mg.
Packaging and storage—
Preserve in tight containers.
Labeling—
Label it to indicate that it is intended for veterinary use only. If it is crystalline, it may be so labeled. If it is intended solely for oral use, it is so labeled. Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
USP Reference standards  11
11 —
—
USP Endotoxin RS
Identification—
A:
To a solution of 4 mg in 2 mL of water, add 0.5 mL of 1 N hydrochloric acid, and heat in a water bath for 20 minutes. Remove the tube from the bath, and add 1.0 mL of a 1 in 200 solution of 1-naphthol in 1 N sodium hydroxide. Heat again for 10 minutes, cool briefly in an ice bath, and add water to make 25 mL: a red color develops, intensifying during about 10 minutes.
B:
A solution (1 in 50) responds to the tests for Sulfate  191
191 .
.
Crystallinity  695
695 (where labeled as being crystalline):
meets the requirements.
(where labeled as being crystalline):
meets the requirements.
pH  791
791 :
between 4.5 and 7.0, in a solution containing 200 mg of dihydrostreptomycin per mL, except that if it is labeled as being solely for oral use, the pH is between 3.0 and 7.0.
:
between 4.5 and 7.0, in a solution containing 200 mg of dihydrostreptomycin per mL, except that if it is labeled as being solely for oral use, the pH is between 3.0 and 7.0.
Loss on drying  731
731 —
Dry about 100 mg in a capillary-stoppered bottle in vacuum at 60
—
Dry about 100 mg in a capillary-stoppered bottle in vacuum at 60 for 3 hours: it loses not more than 5.0% of its weight, except that if it is labeled as being solely for oral use, it loses not more than 14.0% of its weight.
for 3 hours: it loses not more than 5.0% of its weight, except that if it is labeled as being solely for oral use, it loses not more than 14.0% of its weight.
Streptomycin—
Ferric chloride stock solution—
Dissolve 5 g of ferric chloride in 50 mL of 0.1 N hydrochloric acid.
Ferric chloride solution—
Dilute 2.5 mL of Ferric chloride stock solution with sufficient 0.01 N hydrochloric acid to make 100 mL. Use this solution within 1 day.
Standard solutions—
Dissolve an accurately weighed quantity of USP Streptomycin Sulfate RS in water to obtain a stock solution containing 1.0 mg of streptomycin (C21H39N7O12) per mL. Transfer 1.0, 2.0, 3.0, 4.0, and 5.0 mL, respectively, of this stock solution to each of five 25-mL volumetric flasks. Transfer 9.0, 8.0, 7.0, 6.0, and 5.0 mL of water to the flasks, respectively.
Test solution—
Transfer about 800 mg of Dihydrostreptomycin Sulfate, accurately weighed, to a 25-mL volumetric flask, dissolve in and dilute with water to volume, and mix. Transfer 10.0 mL of this solution to a second 25-mL volumetric flask.
Procedure—
To each of the flasks containing the Standard solutions and the Test solution, and to a seventh 25-mL volumetric flask containing 10.0 mL of water to provide a blank, add 2.0 mL of 1 N sodium hydroxide, and heat in a water bath for 10 minutes. Cool the flasks in ice water for 3 minutes, and to each add 2.0 mL of 1.2 N hydrochloric acid and 5.0 mL of Ferric chloride solution. Dilute with water to volume, and mix. Concomitantly determine the absorbances of the solutions from the Standard solutions and the Test solution at the wavelength of maximum absorbance at about 550 nm, with a suitable spectrophotometer, using the blank to set the instrument at zero. Plot the absorbance values of the solutions from the Standard solutions versus concentration, in µg per mL, of streptomycin, and draw the straight line best fitting the five plotted points. From the graph so obtained, determine the concentration, C, in µg per mL, of streptomycin in the solution from the Test solution. Calculate the percentage of streptomycin in the portion of Dihydrostreptomycin Sulfate taken by the formula:
6250C / WP
in which W is the weight, in mg, of Dihydrostreptomycin Sulfate taken, and P is the potency, in µg of dihydrostreptomycin per mg, of the Dihydrostreptomycin Sulfate taken as determined in the Assay: not more than 3.0% is found, except that if it is labeled as being crystalline, not more than 1.0% is found, or if it is labeled as being solely for oral use, not more than 5.0% is found.
Other requirements—
Where the label states that Dihydrostreptomycin Sulfate is sterile, it meets the requirements for Sterility and Bacterial endotoxins under Dihydrostreptomycin Injection. Where the label states that Dihydrostreptomycin Sulfate must be subjected to further processing during the preparation of injectable dosage forms, it meets the requirements for Bacterial endotoxins under Dihydrostreptomycin Injection.
Assay—
Proceed with Dihydrostreptomycin Sulfate as directed for the turbidimetric assay of dihydrostreptomycin under Antibiotics—Microbial Assays  81
81 .
.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Morgan Puderbaugh, B.S.
Associate Scientific Liaison 1-301-998-6833 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2909