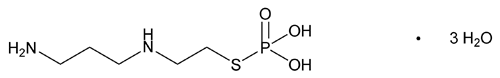
Amifostine
C5H15N2O3PS·3H2O 268.27
Ethanethiol, 2-[(3-aminopropyl)amino]-, dihydrogen phosphate (ester), trihydrate;
S-[2-(3-Aminopropyl)amino]ethyl]dihydrogen phosphorothioate, trihydrate

 [112901-68-5].
[112901-68-5].
(am'' i fos' teen).
C5H15N2O3PS·3H2O 268.27
Ethanethiol, 2-[(3-aminopropyl)amino]-, dihydrogen phosphate (ester), trihydrate;
S-[2-(3-Aminopropyl)amino]ethyl]dihydrogen phosphorothioate, trihydrate
DEFINITION
Amifostine contains NLT 78.0% and NMT 82.0% of C5H15N2O3PS, calculated on the as-is basis.
IDENTIFICATION
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
Buffer:
0.94 g/L of sodium 1-hexanesulfonate. Adjust with phosphoric acid to a pH of 3.0.
Mobile phase:
Methanol and Buffer (7:18)
Standard solution:
3 mg/mL of USP Amifostine RS in water. [Note—Inject immediately after preparation. ]
Sample solution:
3 mg/mL of Amifostine in water.
[Note—Inject immediately after preparation. ]
Chromatographic system
Mode:
LC
Detector:
UV 220 nm
Column:
4.6-mm × 25-cm; 5-µm packing L7
Autosampler temperature:
4
Flow rate:
1.0 mL/min
Injection size:
10 µL
System suitability
Sample:
Standard solution
Suitability requirements
Tailing factor:
NMT 2.0
Column efficiency:
NLT 100 theoretical plates
Relative standard deviation:
NMT 2.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C5H15N2O3PS in the portion of Amifostine taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Amifostine RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Amifostine in the Sample solution (mg/mL) |
Acceptance criteria:
78.0%–82.0% on the as-is basis
IMPURITIES
Inorganic Impurities
• Heavy Metals, Method II  231
231 :
NMT 20 ppm
:
NMT 20 ppm
Organic Impurities
• Procedure
Mobile phase and Chromatographic system:
Proceed as directed in the Assay.
Standard solution:
70 µg/mL of USP Amifostine Thiol RS and 16 µg/mL of USP Amifostine RS in water. [Note—Inject immediately after preparation. ]
System suitability solution:
Use the Standard solution as described in the Assay. [Note—Inject immediately after preparation. ]
Sample solution:
15 mg/mL of Amifostine in water.
[Note—Inject immediately after preparation. ]
System suitability
Samples:
Standard solution and System suitability solution
Suitability requirements
Column efficiency:
NLT 1000 theoretical plates, System suitability solution
Tailing factor:
NMT 2.0, System suitability solution
Relative standard deviation:
NMT 15.0%, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of amifostine thiol in the portion of Amifostine taken:
Result = (rU/rS) × (CS/CU) × (Mr1/Mr2) × 100
| rU | = | = peak response of amifostine thiol from the Sample solution |
| rS | = | = peak response of amifostine thiol from the Standard solution |
| CS | = | = concentration of USP Amifostine Thiol RS in the Standard solution (mg/mL) |
| CU | = | = nominal concentration of amifostine in the Sample solution (mg/mL) |
| Mr1 | = | = molecular weight of amifostine thiol, 134.24 |
| Mr2 | = | = molecular weight of amifostine thiol dihydrochloride, 207.17 |
Calculate the percentage of any other individual impurity in the portion of Amifostine taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of each individual impurity in the Sample solution |
| rS | = | = peak response of amifostine in the Standard solution |
| CS | = | = concentration of USP Amifostine RS in the Standard solution (µg/mL) |
| CU | = | = concentration of the Sample solution (µg/mL) |
Acceptance criteria
Amifostine thiol:
NMT 0.3%
Any individual impurity, excluding amifostine thiol:
NMT 0.1%
Total impurities including amifostine thiol:
NMT 0.3%
SPECIFIC TESTS
• pH  791
791 :
6.5–7.5, in a solution (5 in 100)
:
6.5–7.5, in a solution (5 in 100)
• Water Determination, Method Ic  921
921
Sample solution:
To 100.0 mg of Amifostine, contained in a stoppered centrifuge tube, add 10.0 mL of the solution of N-ethylmaleimide in methanol (4 in 100), and sonicate for 15 min. Shake to disperse, and sonicate for an additional 15 min. Use 1.0 mL of the supernatant.
Acceptance criteria:
19.2%–21.2%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight, light-resistant containers, and store in a refrigerator.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Sujatha Ramakrishna, Ph.D.
Senior Scientific Liaison 1-301-816-8349 |
(SM22010) Monographs - Small Molecules 2 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2156
Pharmacopeial Forum: Volume No. 36(1) Page 62