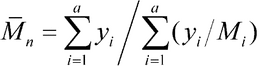Dextran 70
(dex' tran).
Dextrans.
Dextrans.
» Dextran 70 is derived by controlled hydrolysis and fractionation of polysaccharides elaborated by the fermentative action of certain appropriate strains of Leuconostoc mesenteroides (NRRL, B-512F; NCTC, 10817) on a sucrose substrate. It is a glucose polymer in which the linkages between glucose units are almost entirely of the  -1:6 type. Its weight average molecular weight is in the 63,000 to 77,000 range.
-1:6 type. Its weight average molecular weight is in the 63,000 to 77,000 range.
Packaging and storage—
Preserve in well-closed containers. Store at 25 , excursions permitted between 15
, excursions permitted between 15 and 30
and 30 .
.
Labeling—
Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
USP Reference standards  11
11 —
—
USP Dextran 70 RS
USP Dextran 4 Calibration RS
USP Dextran 10 Calibration RS
USP Dextran 40 Calibration RS
USP Dextran 70 Calibration RS
USP Dextran 250 Calibration RS
USP Dextran Vo Marker RS
USP Dextran 70 System Suitability RS
USP Endotoxin RS
Color of solution—
The absorbance of a solution in water (6 in 100), measured in a 4-cm cell determined at 375 nm against a water blank, is not greater than 0.15.
Identification—
B:
Proceed as directed under Dextran 40, except to use Dextran 70 to prepare the Test solutions: the value of the results at the intercept is between 24 and 29 mL per g.
Specific rotation  781S
781S :
between +195
:
between +195 and +203
and +203 .
.
Test solution:
20 mg per mL, heated, if necessary, on a water bath to dissolve.
Bacterial endotoxins  85
85 (where it is labeled as intended for use in the preparation of injectables)—
When tested in Sodium Chloride Injection (0.6 in 10), it contains not more than 0.5 USP Endotoxin Unit per mL.
(where it is labeled as intended for use in the preparation of injectables)—
When tested in Sodium Chloride Injection (0.6 in 10), it contains not more than 0.5 USP Endotoxin Unit per mL.
Safety—
Inject intravenously 1.0 mL of a sterile solution of 6% Dextran 70 in saline TS into each of five mice weighing 18 to 20 g. The injection period is not less than 10 seconds and not greater than 15 seconds. If there are no deaths within 72 hours, it meets the requirements of the test. If 1 or more animals die, continue the test using 10 mice weighing 20 ± 0.5 g. If all animals survive for 72 hours, the requirements of the test are met.
pH  791
791 :
between 4.5 and 7.0, in a solution (6 in 100).
:
between 4.5 and 7.0, in a solution (6 in 100).
Loss on drying  731
731 —
Dry it at 105
—
Dry it at 105 for 5 hours: it loses not more than 7.0% of its weight.
for 5 hours: it loses not more than 7.0% of its weight.
Sulfate  221
221 —
A 1.5-g portion shows no more sulfate than corresponds to 0.45 mL of 0.020 N sulfuric acid (0.03%).
—
A 1.5-g portion shows no more sulfate than corresponds to 0.45 mL of 0.020 N sulfuric acid (0.03%).
Heavy metals, Method II  231
231 :
5 µg per g.
:
5 µg per g.
Limit of nitrogenous impurities (where it is labeled as intended for use in the preparation of injectables)—
Sulfate solution and Indicator—
Proceed as directed under Dextran 40.
Procedure—
Proceed as directed under Dextran 40, except to use 0.2 g of Dextran 70.
Limit of alcohol and related impurities—
Test solution—
Proceed as directed under Dextran 40, except to use 5.0 g of Dextran 70.
Standard solution, Chromatographic system, and Procedure—
Proceed as directed under Dextran 40.
Antigenic impurities (where it is labeled as intended for use in the preparation of injectables)—
Prepare a sterile solution containing 60 mg per mL of Dextran 70 in Sodium Chloride Injection, and proceed as directed under Dextran 40 beginning with “At intervals of about 48 hours.”
Molecular weight distribution and weight and number average molecular weights—
Mobile phase, Calibration solutions, and Marker solution—
Proceed as directed under Dextran 40.
System suitability solution—
Prepare a solution of USP Dextran 70 System Suitability RS in Mobile phase containing 20 mg per mL.
Test solution—
Prepare a solution of Dextran 70 in Mobile phase containing 20 mg per mL.
Chromatographic system (see Chromatography  621
621 )—
Proceed as directed under Dextran 40: Mw for the total molecular weight distribution is between 65,000 and 74,000; Mw for the high-fraction dextran is between 180,000 and 240,000; and Mw for the low-fraction dextran is between 7,000 and 11,000.
)—
Proceed as directed under Dextran 40: Mw for the total molecular weight distribution is between 65,000 and 74,000; Mw for the high-fraction dextran is between 180,000 and 240,000; and Mw for the low-fraction dextran is between 7,000 and 11,000.
Procedure—
Chromatograph a 50-µL volume of the Test solution, and record the peak responses. Calculate values of Mw of the total molecular weight distribution, of the high-fraction dextran, and of the low-fraction dextran as directed for the System suitability solution under Chromatographic system. The Mw values are between 63,000 and 77,000, not more than 195,000, and not less than 13,000, respectively. With the values of b1, b2, b3, b4, and b5, obtained with the Calibration solutions under Chromatographic system, calculate the number average molecular weight, Mn, of the total molecular weight distribution of the Test solution by substituting the corresponding values of Mi, along with their corresponding values of yi, in the equation:
The number average molecular weight, Mn, is between 34,000 and 48,000. Where Dextran 70 is labeled as intended for use in the preparation of injectables, the ratio Mw / Mn is in the 1.4 to 1.9 range.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ritu Agarwal, Ph.D.
Scientific Liaison 1-301-998-6786 |
(BIO12010) Monographs - Biologics and Biotechnology 1 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
|
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology |
USP35–NF30 Page 2856
Pharmacopeial Forum: Volume No. 29(6) Page 1868