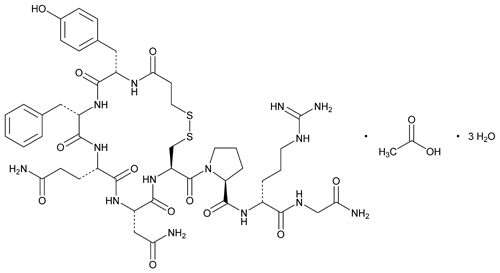
Desmopressin Acetate
(des'' moe pres' in as' e tate).
Vasopressin, 1-(3-mercaptopropanoic acid)-8-d-arginine-, monoacetate (salt).
1-(3-Mercaptopropionic acid)-8-d-arginine-vasopressin monoacetate (salt).
Trihydrate 1183.31
» Desmopressin Acetate is a synthetic octapeptide hormone having the property of antidiuresis. It is a synthetic analog of vasopressin. It contains not less than 95.0 percent and not more than 105.0 percent of desmopressin (C46H64N14O12S2), calculated on the anhydrous, acetic acid-free basis.
Packaging and storage—
Preserve in tight containers, preferably of Type I glass, protected from light. Store at a temperature not exceeding 25 , preferably between 2
, preferably between 2 and 8
and 8 .
.
Labeling—
Label it to state the potency, in mg, of desmopressin.
Identification—
A:
Mass spectral analysis—
Diluent:
a mixture of water and methanol (1:1).
Standard solution—
Dissolve an accurately weighed quantity of USP Desmopressin Acetate RS in Diluent to obtain a solution having a known concentration of about 5 µg per mL.
Test solution—
Dissolve an accurately weighed quantity of Desmopressin Acetate in Diluent to obtain a solution having a known concentration of about 5 µg per mL.
[note—The final concentration of the Standard solution and the Test solution can be adjusted depending on the sensitivity of the mass spectrometer used in the testing. ]
Mass spectrometric system (see Mass Spectrometry  736
736 )—
The LC/MS Spectrometer is equipped with an electrospray interface, positive ion mode, infusion system, and MS/MS capability.
)—
The LC/MS Spectrometer is equipped with an electrospray interface, positive ion mode, infusion system, and MS/MS capability.
Procedure—
Separately infuse the Standard solution and the Test solution at about 5 µL per minute into the mass spectrometer. Obtain optimized MS and MS/MS spectra of the peak with mass-to-charge ratio 1069. For MS spectra, the major peak with mass-to-charge ratio of 1069 should be observed. For MS/MS spectra, product ions at mass-to-charge ratios of about 641, 742, and 995 are present.
B:
The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay.
Specific rotation  781S
781S :
between
:
between  72
72 and
and  82
82 , calculated on the anhydrous, acetic acid-free basis.
, calculated on the anhydrous, acetic acid-free basis.
Test solution:
5 mg per mL, in diluted acetic acid.
Microbial enumeration tests  61
61 and Tests for specified microorganisms
and Tests for specified microorganisms  62
62 —
The total aerobic microbial count does not exceed 100 cfu per g.
—
The total aerobic microbial count does not exceed 100 cfu per g.
Water, Method Ic  921
921 :
not more than 6.0%.
:
not more than 6.0%.
Amino acid content (see Biotechnology-Derived Articles—Amino Acid Analysis  1052
1052 )—
)—
[note—The following method is given for informational purposes; any validated amino acid analysis method can be used. The relative proportions of amino acids, however, must be met for any method used. ]
Solution A—
Prepare a solution having final concentrations of 20 mM sodium acetate, 0.2% (v/v) triethylamine, and 0.3% (v/v) tetrahydrofuran.
Solution B—
Prepare a solution containing 20% (v/v) 100 mM sodium acetate, 40% (v/v) methanol, and 40% (v/v) acetonitrile.
Mobile phase—
Use variable mixtures of Solution A and Solution B as directed for Chromatographic system. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Borate buffer—
Transfer 12.4 g of boric acid to a 500-mL volumetric flask, and suspend it in 300 mL of water. Add 100 mL of 1 N potassium hydroxide, and mix. Adjust with 1 N potassium hydroxide to a pH of 10.4, dilute with water to volume, and mix. Store in a closed plastic container.
Norvaline solution—
Prepare a 4 mM solution of norvaline.
2% DTDPA solution—
Transfer 2 g of dithiodipropionic acid to a 100-mL volumetric flask. Dissolve in and dilute with water to volume.
Sarcosine solution—
Prepare a 4 mM solution of sarcosine.
0.1% Phenol—
Prepare a solution containing 0.1% (w/v) phenol in 6 N hydrochloric acid.
OPA reagent—
Prepare a solution containing 10 mg per mL each of o-phthalaldehyde and 3-mercaptopropionic acid in Borate buffer.
FMOC reagent—
Prepare a solution containing 2.5 mg per mL of 9-fluorenylmethylchloroformate in acetonitrile.
Calibration solution—
Prepare a mixture in which the final concentrations of amino acids are as follows: about 2.50 mM glycine; about 2.50 mM for the l-form of the amino acids lysine, histidine, arginine, aspartic acid, threonine, serine, glutamic acid, proline, alanine, valine, methionine, isoleucine, leucine, tyrosine, and phenylalanine; and about 1.25 mM l-cystine. Transfer a 1-mL aliquot of this solution to a suitable vial, and add 5 µL each of Norvaline solution and 2% DTDPA solution. Evaporate the aliquot to dryness, and add 300 µL of 0.1% Phenol. Mix, and alternate between purging the head space of the vial with nitrogen gas and reducing the pressure to 2 mm of mercury for a total of two purge-vacuum cycles. Purge the sample with nitrogen gas one additional time, and reduce the pressure to 1.5 mm of mercury. Seal, and heat the sample at 110 for 24 hours. Open the vial, and evaporate to dryness. Dissolve the residue in 115 µL of Borate buffer, and add 5 µL of Sarcosine solution. Centrifuge for 2 minutes, and transfer the supernatant to a clean vial. Remove a 6-µL aliquot, and add 1 µL of OPA reagent. Mix, and add 1 µL of FMOC reagent. Mix, add 28 µL of water, and mix again.
for 24 hours. Open the vial, and evaporate to dryness. Dissolve the residue in 115 µL of Borate buffer, and add 5 µL of Sarcosine solution. Centrifuge for 2 minutes, and transfer the supernatant to a clean vial. Remove a 6-µL aliquot, and add 1 µL of OPA reagent. Mix, and add 1 µL of FMOC reagent. Mix, add 28 µL of water, and mix again.
Test solution—
Dissolve an accurately weighed quantity of Desmopressin Acetate in water to obtain a solution having a known concentration of about 1.00 mg per mL. Add 5 µL each of Norvaline solution and 2% DTDPA solution to a 1-mL aliquot, and prepare as directed in Calibration solution, beginning with “Bring the aliquot to dryness and add 300 µL of 0.1% Phenol.”
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a thermoregulated autosampler, set at 4
)—
The liquid chromatograph is equipped with a thermoregulated autosampler, set at 4 , that is capable of adding and mixing derivatizing agents; a multi-wavelength detector set at 262 nm and 338 nm; and a 2.1-mm × 20-cm column that contains 5-µm packing L1. The flow rate is about 0.45 mL per minute. The column temperature is maintained at 40
, that is capable of adding and mixing derivatizing agents; a multi-wavelength detector set at 262 nm and 338 nm; and a 2.1-mm × 20-cm column that contains 5-µm packing L1. The flow rate is about 0.45 mL per minute. The column temperature is maintained at 40 . The chromatograph is programmed as follows.
. The chromatograph is programmed as follows.
Process and inject the Calibration solution and the Test solution, and record the peak responses for individual amino acid derivatives as directed for Procedure: the order of elution of the amino acid derivatives is aspartic acid, glutamic acid, serine, histidine, glycine, threonine, cysteine (reduced cystine), alanine, arginine, tyrosine, valine, methionine, norvaline, phenylalanine, isoleucine, leucine, lysine, and proline; the resolution, R, between the amino acid pairs histidine and glycine, alanine and arginine, and valine and methionine in the Calibration solution is not less than 1; and the relative standard deviation for triplicate injection of the Calibration solution is not greater than 15% for cysteine, lysine and proline, and not greater than 10% for all other amino acids. The peak area of the norvaline peak in the Test solution should be not less than 80% and not greater than 120% of that found in the Calibration solution.
| Time (minutes) |
Solution A
(%) |
Solution B
(%) |
Elution |
|---|---|---|---|
| 0 | 100 | 0 | equilibration |
| 0–17 | 100®40 | 0®60 | linear gradient |
| 17–18.10 | 40®0 | 60®100 | linear gradient |
| 18.10–24 | 0 | 100 | isocratic |
| 24–25 | 0®100 | 100®0 | linear gradient |
| 25–35 | 100 | 0 | re-equilibration |
Procedure—
Using the autosampler, separately remove equal volumes (about 6 µL) of the Calibration solution and the Test solution, and to each add 1 µL of OPA reagent. Mix, and to each add 1 µL of FMOC reagent. Mix, add 28 µL of water to each, mix again, and inject the entire volume into the chromatograph. Record the area responses for the main peaks, and identify the amino acids. Using the Calibration solution as a standard, express the content of each amino acid in moles. With the content for arginine set to 1, calculate the relative proportions of the amino acids: aspartic acid, glutamic acid, proline, glycine, and phenylalanine are between 0.95 and 1.05; tyrosine is between 0.7 and 1.05; cysteine is between 0.30 and 1.05; lysine, isoleucine, and leucine are absent; and not more than traces of other amino acids are found.
Limit of acetic acid—
Internal standard solution—
Transfer about 16 mL of hydrochloric acid into a 1000-mL volumetric flask containing about 500 mL of water, and mix. Add about 0.5 mL of propionic acid, accurately measured, dilute with acetonitrile to volume, and mix.
Standard solution—
Transfer about 1.049 g of acetic acid, accurately measured, to a 100-mL volumetric flask. Dilute with Internal standard solution to volume, and mix. Transfer 2.5 mL of the resulting solution to a 50-mL volumetric flask. Dilute with Internal standard solution to volume, and mix.
Test solution—
Dissolve about 5 mg of Desmopressin Acetate, accurately weighed, in 0.5 mL of Internal standard solution, and mix.
Chromatographic system (see Chromatography  621
621 )—
The gas chromatograph is equipped with a flame-ionization detector and a 0.32-mm × 30-m fused silica capillary column coated with a 0.25-µm film of phase G35. The carrier gas is helium, flowing at a rate of about 3 mL per minute, and the split flow ratio is 20:3. The column temperature is maintained at 120
)—
The gas chromatograph is equipped with a flame-ionization detector and a 0.32-mm × 30-m fused silica capillary column coated with a 0.25-µm film of phase G35. The carrier gas is helium, flowing at a rate of about 3 mL per minute, and the split flow ratio is 20:3. The column temperature is maintained at 120 , and the injection port and detector temperatures are maintained at 250
, and the injection port and detector temperatures are maintained at 250 . Chromatograph six replicate injections of the Standard solution, and record the peak areas as directed for Procedure: the order of elution is acetic acid followed by propionic acid; the resolution, R, between acetic acid and propionic acid is not less than 5.0; the tailing factor for acetic acid is not more than 3.0; and the relative standard deviation of the peak area ratio of acetic acid to propionic acid is not more than 15%.
. Chromatograph six replicate injections of the Standard solution, and record the peak areas as directed for Procedure: the order of elution is acetic acid followed by propionic acid; the resolution, R, between acetic acid and propionic acid is not less than 5.0; the tailing factor for acetic acid is not more than 3.0; and the relative standard deviation of the peak area ratio of acetic acid to propionic acid is not more than 15%.
Procedure—
Separately inject equal volumes (about 1.0 µL) of the Standard solution and the Test solution into the chromatograph, and measure the peak responses. Calculate the percentage of acetic acid in the portion of Desmopressin Acetate taken by the formula:
100(CS/CU)(RU/RS)
in which CS is the concentration, in mg per mL, of acetic acid in the Standard solution; CU is the concentration, in mg per mL, of desmopressin acetate in the Test solution; and RU and RS are the peak area ratios of acetic acid to the internal standard obtained from the Test solution and the Standard solution, respectively: not less than 3% and not more than 8.0% is found.
Chromatographic purity—
Mobile phase and System suitability solution—
Proceed as directed in the Assay.
Standard solution—
Dissolve an accurately weighed quantity of USP Desmopressin Acetate RS in Mobile phase to obtain a solution having a known concentration of about 1 µg per mL.
Test solution—
Dissolve an accurately weighed quantity of Desmopressin Acetate in Mobile phase to prepare a solution having a known concentration of about 200 µg per mL.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 220-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L1. The flow rate is about 1.0 mL per minute, and the column is maintained at 30
)—
The liquid chromatograph is equipped with a 220-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L1. The flow rate is about 1.0 mL per minute, and the column is maintained at 30 . Chromatograph the Standard solution and the System suitability solution, and record the peak areas as directed for Procedure: the desmopressin peak elutes before the oxytocin peak; the resolution, R, between desmopressin and oxytocin is not less than 1.5; the tailing factor is not greater than 2.0; and the relative standard deviation of the desmopressin peak area for replicate injections of the Standard solution is not greater than 5.0%.
. Chromatograph the Standard solution and the System suitability solution, and record the peak areas as directed for Procedure: the desmopressin peak elutes before the oxytocin peak; the resolution, R, between desmopressin and oxytocin is not less than 1.5; the tailing factor is not greater than 2.0; and the relative standard deviation of the desmopressin peak area for replicate injections of the Standard solution is not greater than 5.0%.
Procedure—
Separately inject equal volumes (about 200 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the response for each peak, except for the main desmopressin peak in the chromatogram of the Test solution. Calculate the percentage of each impurity in the portion of Desmopressin Acetate taken by the formula:
100(CS/CU)(ri/rS)
in which CS is the concentration, in mg per mL, of USP Desmopressin Acetate RS, calculated on the anhydrous, acetic acid-free basis, in the Standard solution; CU is the concentration, in mg per mL, of Desmopressin Acetate, calculated on the anhydrous, acetic acid-free basis, in the Test solution; ri is the peak response of an individual impurity in the chromatogram obtained from the Test solution; and rS is the desmopressin peak response obtained from the Standard solution: not more than 0.5% of any individual impurity is found, and the sum of all impurities is less than 1.5%.
Assay—
Buffer solution—
Dissolve 3.4 g of monobasic potassium phosphate and 2.0 g of sodium 1-heptanesulfonic acid in 1000 mL of water. Adjust the pH to 4.50 ± 0.05 with phosphoric acid or sodium hydroxide, as needed. Pass through a filter having a porosity of 0.45-µm.
Mobile phase—
Mix 780 mL of Buffer solution with 220 mL of acetonitrile, and degas. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ). [note—The retention time of desmopressin is very sensitive to the composition of the Mobile phase. ]
). [note—The retention time of desmopressin is very sensitive to the composition of the Mobile phase. ]
Standard preparation—
Dissolve an accurately weighed quantity of USP Desmopressin Acetate RS in Mobile phase to obtain a solution having a known concentration of about 20 µg per mL.
Assay preparation—
Dissolve an accurately weighed quantity of Desmopressin Acetate in Mobile phase to prepare a solution having a known concentration of about 20 µg per mL.
System suitability solution—
Dissolve about 1 mg of oxytocin, accurately weighed, in a 50-mL volumetric flask, dilute with Mobile phase to volume, and mix. Transfer 5.0 mL of the resulting solution and 5.0 mL of Assay preparation to a 100-mL volumetric flask, dilute with Mobile phase to volume, and mix.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 220-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L1. The column temperature is maintained at 30
)—
The liquid chromatograph is equipped with a 220-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L1. The column temperature is maintained at 30 . The flow rate is about 1.0 mL per minute. Chromatograph the Standard preparation and the System suitability solution, and record the peak areas as directed for Procedure: the desmopressin peak elutes before the oxytocin peak; the resolution, R, between desmopressin and oxytocin is not less than 1.5; the tailing factor is not greater than 2.0; and the relative standard deviation of the desmopressin peak area for replicate injections is not greater than 2.0%.
. The flow rate is about 1.0 mL per minute. Chromatograph the Standard preparation and the System suitability solution, and record the peak areas as directed for Procedure: the desmopressin peak elutes before the oxytocin peak; the resolution, R, between desmopressin and oxytocin is not less than 1.5; the tailing factor is not greater than 2.0; and the relative standard deviation of the desmopressin peak area for replicate injections is not greater than 2.0%.
Procedure—
Separately inject equal volumes (about 50 µL) of the Standard preparation and the Assay preparation, both freshly prepared, into the chromatograph, record the chromatograms, and measure the desmopressin peak areas. Calculate the percentage of C46H64N14O12S2 in the portion of Desmopressin Acetate taken by the formula:
100(CS/CU)(rU/rS)
in which CS is the concentration, in mg per mL, of USP Desmopressin Acetate RS, calculated on the anhydrous, acetic acid-free basis, in the Standard preparation; CU is the concentration, in mg per mL, of Desmopressin Acetate, calculated on the anhydrous, acetic acid-free basis, in the Assay preparation; and rU and rS are the peak responses for desmopressin obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Thomas A. Sigambris, M.S.
Scientific Liaison 1-301-998-6789 |
(BIO12010) Monographs - Biologics and Biotechnology 1 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
|
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology |
USP35–NF30 Page 2827
Pharmacopeial Forum: Volume No. 31(4) Page 1052