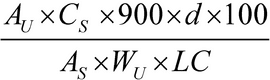Meloxicam Oral Suspension
» Meloxicam Oral Suspension contains not less than 90.0 percent and not more than 110.0 percent of the labeled amount of meloxicam (C14H13N3O4S2).
Packaging and storage—
Preserve in well-closed containers. Store at 25 , excursions permitted between 15
, excursions permitted between 15 and 30
and 30 .
.
Identification—
Test solution—
Transfer a volume of Oral Suspension, equivalent to about 2.5 mg of meloxicam, to a 10-mL volumetric flask. Dilute with acetone to volume, and mix for 10 minutes. If necessary, pass through fluted filter paper.
Standard solution:
0.25 mg per mL, prepared by dissolving USP Meloxicam RS in 1 mL of water and diluting with acetone to volume.
Developing solvent solution:
a mixture of chloroform, methanol, and ammonium hydroxide (80:20:1)
Procedure—
Proceed as directed in the chapter. After removing the plate from the chamber and drying, examine the chromatograms under UV light at 254-nm: the RF value (approximately 0.45) of the principal dark spot obtained from the Test solution corresponds to that obtained from the Standard solution.
B:
The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay.
pH  791
791 :
between 3.5 and 4.5.
:
between 3.5 and 4.5.
Viscosity  911
911 —
Determine using a shear rate programmable rotational viscometer: between 40 and 100 centipoises, determined at 20
—
Determine using a shear rate programmable rotational viscometer: between 40 and 100 centipoises, determined at 20 .
.
Dissolution  711
711 —
—
Medium:
pH 7.5 phosphate buffer; 900 mL.
Apparatus 2:
25 rpm.
Time:
15 minutes.
Determine the amount of C14H13N3O4S2 dissolved by employing the following method.
Standard solution—
Transfer about 20.83 mg of USP Meloxicam RS, accurately weighed, into a 100-mL volumetric flask. Dissolve in 5 mL of methanol and 1 mL of 0.1 M sodium hydroxide, and dilute with Medium to volume. Dilute with Medium to a final concentration of about 8.3 µg per mL of meloxicam.
Test solution—
Shake each sample for 15 minutes. Weigh six portions, equivalent to 7.5 mg of the Oral Suspension, into separate tared 10-mL beakers, and record each weight. Introduce each of the samples to the middle of the dissolution vessels, and rinse each beaker with about 20 mL of the Medium withdrawn from the vessel. Carefully lower the paddle to the appropriate height and start the rotation. After completion of the dissolution, pass a 20-mL aliquot through a nylon filter having a 0.45-µm porosity, discarding the first 3 mL of the filtrate.
Procedure—
Determine the amount of C14H13N3O4S2 dissolved by employing UV absorption at the wavelength of maximum absorbance at about 362 nm on the Test solution in comparison with the Standard solution, using Medium as the blank. Calculate the percentage of C14H13N3O4S2 released by the formula:
in which AU and AS are the absorbances obtained from the Test solution and the Standard solution, respectively; CS is the concentration, in mg per mL, of the Standard solution; d is the density, in g per mL, of the Oral Suspension; WU is the weight, in mg, of the Oral Suspension taken; 900 is the volume, in mL of the Medium; 100 is the conversion factor to percentage; and LC is the label claim, in mg per mL.
Tolerances—
Not less than 75% (Q) of the labeled amount of C14H13N3O4S2 is dissolved in 15 minutes.
Microbial enumeration tests  61
61 and Tests for specified microorganisms
and Tests for specified microorganisms  62
62 —
The total aerobic microbial count does not exceed 100 cfu per g or 100 cfu per mL. The total yeasts and molds count does not exceed 50 cfu per g or 50 cfu per mL. It meets the requirements of the test for the absence of Escherichia coli.
—
The total aerobic microbial count does not exceed 100 cfu per g or 100 cfu per mL. The total yeasts and molds count does not exceed 50 cfu per g or 50 cfu per mL. It meets the requirements of the test for the absence of Escherichia coli.
Chromatographic purity—
Buffer, Mobile phase, and Diluent—
Proceed as directed in the Assay.
Related compound standard stock solution—
Proceed as directed for Related compound standard stock preparation in the Assay.
Sensitivity solution
—Dilute the Related compound standard stock solution with Diluent to a final concentration of about 0.08 µg per mL.
Related compound standard solution—
Dilute Related compound standard stock preparation with Diluent to a final concentration of about 0.5 µg per mL.
Test solution—
Proceed as directed for Assay preparation in the Assay.
Chromatographic system (see Chromatography  621
621 )—
Proceed as directed in the Assay. Chromatograph the Sensitivity solution (about 10 µL), and record the peak responses as directed for Procedure at 260 nm: the relative standard deviation of three replicate injections is not more than 10% for meloxicam related compound B. Chromatograph the Related compound standard solution (about 10 µL), and record the peak responses as directed for Procedure at 260 nm: the tailing factor for meloxicam related compound B is not more than 2.0.
)—
Proceed as directed in the Assay. Chromatograph the Sensitivity solution (about 10 µL), and record the peak responses as directed for Procedure at 260 nm: the relative standard deviation of three replicate injections is not more than 10% for meloxicam related compound B. Chromatograph the Related compound standard solution (about 10 µL), and record the peak responses as directed for Procedure at 260 nm: the tailing factor for meloxicam related compound B is not more than 2.0.
Procedure—
Separately inject equal volumes (about 10 µL) of the Related compound standard solution and the Test solution into the chromatograph, record the chromatograms, and record the peak areas at 260 nm and 360 nm. The run time is about 20 minutes or two times the retention time of meloxicam. Calculate the percentage of meloxicam related compound B in the portion of Oral Suspension taken by the formula:
(5000/L)(C/V)(rU / rS)
in which L is the label claim, in mg per mL; C is the concentration, in mg per mL, of USP Meloxicam Related Compound B RS in the Related compound standard solution; V is the volume, in mL, of Oral Suspension taken to prepare the Test solution; rU is the peak area obtained for meloxicam related compound B in the Test solution at 260 nm; and rS is the peak area for meloxicam related compound B in the Related compound standard solution at 260 nm. Calculate the percentage of each unknown degradation product in the portion of Oral Suspension taken by the formula:
100(ri / rs)
in which ri is the area of any unknown degradant at 360 nm; rs is the sum of areas of meloxicam and all impurities in the Test solution at 360 nm. Not more than 0.15% of meloxicam related compound B is found; not more than 0.2% of any individual unknown degradation product is found; and not more than 0.5% of total degradation products is found.
Assay—
Buffer—
Dissolve 2 g of monohydrate citric acid and 2 g of boric acid in 1000 mL of water, and adjust with dihydrate trisodium citrate to a pH of 2.9.
Mobile phase—
Mix 565 mL of Buffer, 260 mL of methanol, and 200 mL of acetonitrile. Degas the solution, and then dissolve 200 mg of sodium dodecyl sulfate in 1000 mL of the resulting solution.
Diluent—
Dissolve 3 g of boric acid and 1.5 g of dihydrate trisodium citrate in 1000 mL of water, and adjust with 2 M sodium hydroxide to a pH of 8.3. Mix 420 mL of the resulting buffer with 420 mL of methanol and 160 mL of acetonitrile.
Standard stock preparation—
Transfer about 67 mg of USP Meloxicam RS, accurately weighed, into a 100-mL volumetric flask. Add 3.0 mL of dimethylformamide. Swirl the flask, and allow to stand for about 5 minutes. Add 15 mL of methanol. Dilute with Diluent to just below volume. Sonicate for 30 minutes, and mix until dissolved. Cool to room temperature. Dilute with Diluent to volume.
Standard preparation—
Dilute Standard stock preparation with Diluent to a final concentration of about 0.27 mg per mL.
Related compound standard stock preparation—
Transfer about 21 mg of USP Meloxicam Related Compound B RS, accurately weighed, into a 100-mL volumetric flask. Add 3.0 mL of dimethylformamide, 15 mL of methanol, and about 60 mL of Diluent. Sonicate, and mix until dissolved. Cool to room temperature. Dilute with Diluent to volume. Dilute further with Diluent to a concentration of about 8.4 µg per mL.
System suitability solution—
Transfer a volume of Oral Suspension, equivalent to about 15 mg of meloxicam, accurately weighed, to a 50-mL volumetric flask. Add 3.0 mL of Related compound standard stock preparation. Add 3.0 mL of dimethylformamide. Swirl the flask, and allow to stand for about 5 minutes. Add 15 mL of methanol. Dilute with Diluent to just below volume. Sonicate for 30 minutes, mixing the flask vigorously about every 5 minutes. Cool to room temperature. Dilute with Diluent to volume. Mix, and allow particulates to settle. Pass through a 0.45-µm membrane filter with a fiberglass prefilter.
Assay preparation—
Transfer an accurately meaured volume of Oral Suspension, equivalent to about 15 mg of meloxicam, to a 50-mL volumetric flask. Add 3.0 mL of dimethylformamide. Swirl the flask, and allow to stand for about 5 minutes. Add 15 mL of methanol. Dilute with Diluent to just below volume. Sonicate for 30 minutes, mixing the flask vigorously about every 5 minutes. Cool to room temperature. Dilute with Diluent to volume. Mix, and allow particulates to settle. Pass through a 0.45-µm membrane filter with a fiberglass prefilter.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a programmable dual wavelength detector, a single wavelength detector in series, or a photodiode array detector capable of detecting wavelengths from 190 nm to 400 nm, or equivalent, and a 4-mm × 12.5-cm analytical column that contains 5-µm packing L1. The column temperature is maintained at 40
)—
The liquid chromatograph is equipped with a programmable dual wavelength detector, a single wavelength detector in series, or a photodiode array detector capable of detecting wavelengths from 190 nm to 400 nm, or equivalent, and a 4-mm × 12.5-cm analytical column that contains 5-µm packing L1. The column temperature is maintained at 40 . The flow rate is about 1.0 mL per minute. The run time is about 20 minutes or two times the retention time of meloxicam. Chromatograph the System suitability solution (about 10 µL), and record the peak responses as directed for Procedure at 360 nm and 260 nm: at 360 nm the resolution, R, between meloxicam and any other adjacent peak is not less than 1.5. The tailing factor for the meloxicam peak is not more than 2.0. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure at 360 nm: the relative standard deviation for replicate injections of the Standard preparation is not more than 1.5%.
. The flow rate is about 1.0 mL per minute. The run time is about 20 minutes or two times the retention time of meloxicam. Chromatograph the System suitability solution (about 10 µL), and record the peak responses as directed for Procedure at 360 nm and 260 nm: at 360 nm the resolution, R, between meloxicam and any other adjacent peak is not less than 1.5. The tailing factor for the meloxicam peak is not more than 2.0. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure at 360 nm: the relative standard deviation for replicate injections of the Standard preparation is not more than 1.5%.
Procedure—
Separately inject equal volumes (about 10 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and record the peak areas at 360 nm. Calculate the amount of meloxicam (C14H13N3O4S2), in mg per mL, in the portion of Oral Suspension taken by the formula:
50(C/V)(rU / rS)
in which C is the concentration, in mg per mL, of USP Meloxicam RS in the Standard preparation; V is the volume, in mL, of Oral Suspension taken to prepare the Assay preparation; rU is the peak area obtained for meloxicam in the Assay preparation at 360 nm; and rS is the peak area for meloxicam in the Standard solution at 360 nm.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Clydewyn M. Anthony, Ph.D.
Senior Scientific Liaison 1-301-816-8139 |
(SM22010) Monographs - Small Molecules 2 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
|
| Margareth R.C. Marques, Ph.D.
Senior Scientific Liaison 1-301-816-8106 |
(GCDF2010) General Chapters - Dosage Forms | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology |
USP35–NF30 Page 3791
Pharmacopeial Forum: Volume No. 32(6) Page 1721