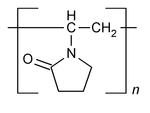
Crospovidone
(kros poe' vi done).
Portions of the monograph text that are national USP text, and are not part of the harmonized text, are marked with symbols (
 ) to specify this fact.
) to specify this fact.
DEFINITION
Crospovidone is a water-insoluble synthetic cross-linked homopolymer of N-vinyl-2-pyrrolidinone. It contains NLT 11.0% and NMT 12.8% of nitrogen (N), calculated on the dried basis. Two types of Crospovidone are available, depending on the particle size: Type A and Type B.
IDENTIFICATION
•  A. Infrared Absorption
A. Infrared Absorption  197K
197K :
Previously dried in a vacuum at 105
:
Previously dried in a vacuum at 105 for 1 h
for 1 h
• B.
Sample:
1 g
Analysis:
Suspend the Sample in 10 mL of water, add 0.1 mL of 0.1 N iodine, and shake for 30 s. Add 1 mL of starch TS, and shake.
Acceptance criteria:
No blue color develops.
• C.
To 10 mL of water add 0.1 g and shake. A suspension is formed, and no clear solution is obtained within 15 min.
• D.
Sample:
20 g of the dried substance
Analysis:
Clean and dry the analytical sieves used in the analysis by washing the sieves in hot water. Allow to dry overnight in a drying cabinet at 105 . Place the Sample in a 1000-mL conical flask, add 500 mL of water, and shake the suspension for 30 min. Pour the suspension through a 63-µm analytical sieve, previously tared, and rinse the sieve with water until the filtrate is clear. Dry the sieve and sample residue at 105
. Place the Sample in a 1000-mL conical flask, add 500 mL of water, and shake the suspension for 30 min. Pour the suspension through a 63-µm analytical sieve, previously tared, and rinse the sieve with water until the filtrate is clear. Dry the sieve and sample residue at 105 for 5 h in a drying cabinet without circulating air. Cool in a desiccator for 30 min, and weigh.
for 5 h in a drying cabinet without circulating air. Cool in a desiccator for 30 min, and weigh.
Calculate the percentage sieving residue fraction of sample particles having a diameter of more than 63 µm:
Result = [(m1  m3) × 100]/m2
m3) × 100]/m2
| m1 | = | = mass of the sieve and sample residue, after drying for 5 h (g) |
| m3 | = | = mass of the sieve (g) |
| m2 | = | = initial mass of the sample, calculated on a dried basis (g) |
Acceptance criteria:
If the sieving residue fraction is more than 15%, the substance is classified as Type A; if the sieving residue fraction is NMT 15%, the substance is classified as Type B.
ASSAY
• Nitrogen Determination, Method II  461
461
Sample:
0.1 g
Analysis:
Proceed as directed, using the Sample. In the Procedure, omit the use of hydrogen peroxide, and use 5 g of a powdered mixture of potassium sulfate, cupric sulfate, and titanium dioxide (33:1:1), instead of potassium sulfate and cupric sulfate (10:1). Heat until a clear, light green solution is obtained. Heat for an additional 45 min, and proceed as directed for Procedure, beginning with “Cautiously add to the digestion mixture 70 mL of water”.
Acceptance criteria:
11.0%–12.8% on the dried basis
IMPURITIES
• Residue on Ignition  281
281 :
NMT 0.1%, determined on 1.0 g
:
NMT 0.1%, determined on 1.0 g
•  Heavy Metals, Method II
Heavy Metals, Method II  231
231 :
NMT 10 ppm
:
NMT 10 ppm
• Peroxides
Sample suspension A:
[Note—Use for Type A. ]
40 mg/mL in water. To 25 mL of this suspension add 2 mL of titanium trichloride–sulfuric acid TS. Allow to stand for 30 min, and filter.
Sample suspension B:
[Note—Use for Type B. ]
16 mg/mL in water. To 25 mL of this suspension add 2 mL of titanium trichloride–sulfuric acid TS. Allow to stand for 30 min, and filter.
Compensation liquid A:
[Note—Use for Type A. ]
40 mg/mL in water. Filter, take 25 mL, and add 2 mL of a 13% solution of sulfuric acid.
Compensation liquid B:
[Note—Use for Type B. ]
16 mg/mL in water. Filter, take 25 mL, and add 2 mL of a 13% solution of sulfuric acid.
Analysis:
Measure the absorbance of the filtrate at 405 nm against the appropriate compensation liquid.
Acceptance criteria:
NMT 0.35. For Type A, this corresponds to NMT 400 ppm expressed as H2O2; for Type B, this corresponds to NMT 1000 ppm expressed as H2O2.
• Vinylpyrrolidinone
Mobile phase:
Acetonitrile and water (1:9)
Sample solution:
25 mg/mL of suspension in methanol. Shake for 60 min. Leave the bulk to settle, and pass through a filter of 0.2-µm pore size.
Reference stock solution A:
5 µg/mL of vinylpyrrolidinone in methanol
Reference stock solution B:
100 µg/mL of vinylpyrrolidinone and 5 mg/mL of vinyl acetate in methanol
Reference solution A:
A 1-in-20 solution of Reference stock solution A in Mobile phase
Reference solution B:
A 1-in-100 solution of Reference stock solution B in Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV
Analytical wavelength:
235 nm
Precolumn:
4-mm × 2.5-cm; 5-µm packing L1
Column:
4-mm × 25-cm; 5-µm packing L1
Column temperature:
40
Flow rate:
1 mL/min
Injection size:
50 µL. [Note—After each injection of the Sample solution, wash the precolumn by passing the Mobile phase backwards, at the same flow rate as applied in the test, for 30 min. ]
System suitability
Samples:
Reference solution A and Reference solution B
Suitability requirements
Resolution:
NLT 2.0 between vinylpyrrolidone and vinyl acetate, Reference solution B
Relative standard deviation:
NMT 2.0% for 6 injections, Reference solution A
Analysis
Samples:
Sample solution and Reference solution A
Record the chromatograms, and measure the responses for the vinylpyrrolidinone peak.
Acceptance criteria:
The area of the peak from the Sample solution is NMT the area of the principal peak from Reference solution A (NMT 10 ppm).
SPECIFIC TESTS
• Loss on Drying  731
731 :
Dry 0.5 g at 105
:
Dry 0.5 g at 105 to constant weight: it loses NMT 5.0% of its weight.
to constant weight: it loses NMT 5.0% of its weight.
• Water-Soluble Substances
Sample:
25.0 g
Analysis:
Transfer the Sample to a 400-mL beaker, add 200 mL of water, and stir on a magnetic stirrer, using a 5-cm stirring bar, for 1 h. Transfer to a 250-mL volumetric flask with the aid of 25 mL of water. Add water to volume. Allow the bulk of the solids to settle. Pass 100 mL of the relatively clear supernatant through a membrane filter of 0.45-µm pore size, protected against clogging by superimposing a membrane filter of 3-µm pore size. While filtering, stir the solution above the filter manually or with a mechanical stirrer, taking care not to physically damage the membrane filter. Transfer 50.0 mL of the clear filtrate to a tared 100-mL beaker, evaporate to dryness, and dry at 110 for 3 h.
for 3 h.
Acceptance criteria:
The weight of the residue does not exceed 75 mg (1.5%).
ADDITIONAL REQUIREMENTS
•  Packaging and Storage:
Preserve in tight containers.
Packaging and Storage:
Preserve in tight containers.
•  Labeling:
The label states the type (Type A or Type B).
Labeling:
The label states the type (Type A or Type B).
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Robert H. Lafaver, M.S.
Scientific Liaison 1-301-816-8335 |
(EXC2010) Monographs - Excipients |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 1775
Pharmacopeial Forum: Volume No. 35(3) Page 671