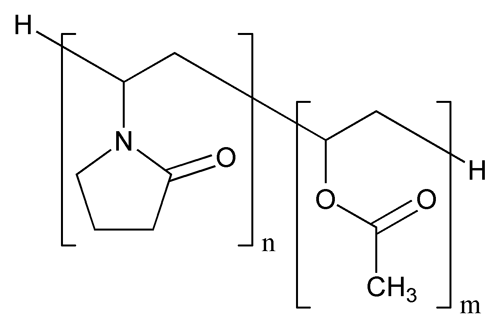
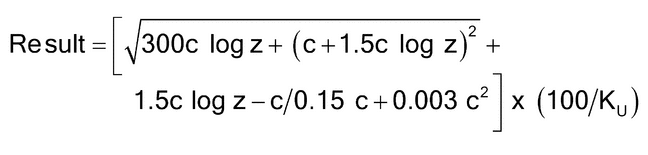Copovidone
(C6H9NO)n + (C4H6O2)m
Acetic acid ethenyl ester polymer with 1-ethenyl-2-pyrrolidone;
1-Vinyl-2-pyrrolidone polymer with vinyl acetate

 [25086-89-9].
[25086-89-9].
(koe' poe vi done).
(C6H9NO)n + (C4H6O2)m
Acetic acid ethenyl ester polymer with 1-ethenyl-2-pyrrolidone;
1-Vinyl-2-pyrrolidone polymer with vinyl acetate
DEFINITION
Copovidone is a copolymer of 1-vinyl-2-pyrrolidone and vinyl acetate in the mass proportion of 3:2. The nominal K-value of Copovidone as stated in the labeling is NLT 90.0% and NMT 110.0%.
IDENTIFICATION
• B. Procedure
Analysis:
To 5 mL of a 20-mg/mL solution, add a few drops of iodine TS.
Acceptance criteria:
A deep red color is produced.
ASSAY
• K-value
Sample solution:
Transfer a quantity of undried Copovidone, equivalent to 1.0 g on the dried basis, to a 100-mL volumetric flask, and dissolve in and dilute with water to volume. Allow to stand for 1 h.
Analysis:
Determine the viscosity, using a capillary-tube viscosimeter (see Viscosity  911
911 ), of this solution at 25 ± 0.2
), of this solution at 25 ± 0.2 . Calculate the relative K-value of Copovidone:
. Calculate the relative K-value of Copovidone:
| c | = | = weight on the dried basis, of the specimen tested in each 100.0 mL of solution (g) |
| z | = | = viscosity of the Sample solution relative to that of water |
| Ku | = | = nominal K-value stated on the label |
Acceptance criteria:
90.0%–110.0%
OTHER COMPONENTS
• Procedure 1: Content of Copolymerized Vinyl Acetate
Analysis:
Determine the saponification value as directed under Fats and Fixed Oils  401
401 , Saponification Value.
, Saponification Value.
Calculate the percentage of copolymerized vinyl acetate in the portion of Copovidone taken:
Result = 0.1 × (Mr1/Mr2) × S
| Mr1 | = | = molecular weight of vinyl acetate, 86.09 |
| Mr2 | = | = molecular weight of potassium hydroxide, 56.11 |
| S | = | = saponification value |
Acceptance criteria:
35.3%–41.4% of the copolymerized vinyl acetate component, calculated on the dried basis
• Procedure 2: Nitrogen Determination, Method II  461
461
Analysis:
Proceed as directed using 0.1 g of Copovidone. In the procedure, use 5 g of a powdered mixture of potassium sulfate, cupric sulfate, and titanium dioxide (33:1:1) instead of potassium sulfate and cupric sulfate (10:1); omit the use of hydrogen peroxide; and heat until the solution has a clear, yellow-green color and the sides of the flask are free from carbonaceous material. Then heat for a further 45 min; add 20 mL of water, instead of 70 mL, after the second heating; and use bromocresol green–methyl red TS instead of methyl red–methylene blue TS. Titrate the distillate with 0.05 N sulfuric acid VS until the color of the solution changes from green through pale grayish blue to pale grayish red-purple.
Acceptance criteria:
7.0%–8.0%, on the dried basis
IMPURITIES
Inorganic Impurities
• Residue on Ignition  281
281 :
NMT 0.1%
:
NMT 0.1%
Organic Impurities
• Procedure 1: Limit of Aldehydes
Solution A:
17.4 mg/mL of monobasic potassium phosphate, adjusted if necessary, with 1 N potassium hydroxide to a pH of 9.0.
Solution B:
Transfer a quantity of lyophilized aldehyde dehydrogenase equivalent to 70 units to a glass vial, and dissolve in 10.0 mL of water. [Note—This solution is stable for 8 h at 4 . ]
. ]
Solution C:
40 mg of nicotinamide adenine dinucleotide in 10 mL of Solution A, in a glass vial. [Note—This solution is stable for 4 weeks at 4 . ]
. ]
Blank solution:
Water
Standard solution:
Transfer 2 mL of water at 4 to a glass weighing bottle, and weigh. Add 100 mg of freshly distilled acetaldehyde, and weigh. Transfer this solution to a 100-mL volumetric flask. Rinse the weighing bottle with several portions of water at 4
to a glass weighing bottle, and weigh. Add 100 mg of freshly distilled acetaldehyde, and weigh. Transfer this solution to a 100-mL volumetric flask. Rinse the weighing bottle with several portions of water at 4 , and transfer each rinsing to the 100-mL volumetric flask. Dilute the solution in the 100-mL volumetric flask with water at 4
, and transfer each rinsing to the 100-mL volumetric flask. Dilute the solution in the 100-mL volumetric flask with water at 4 to volume. Store at 4
to volume. Store at 4 for 20 h. Transfer 1 mL of this solution to a 100-mL volumetric flask, and dilute with Solution A to volume.
for 20 h. Transfer 1 mL of this solution to a 100-mL volumetric flask, and dilute with Solution A to volume.
Sample solution:
10 mg/mL of Copovidone in Solution A, in a 100-mL volumetric flask. Insert a stopper into the flask, heat at 60 for 1 h, and cool to room temperature.
for 1 h, and cool to room temperature.
Analysis:
Pipet 0.5 mL each of the Standard solution, the Sample solution, and the Blank solution into separate 1-cm cells. Add 2.5 mL of Solution A and 0.2 mL of Solution C to each cell. Cover the cells to exclude oxygen. Mix by inversion, and allow to stand for 2–3 min at 22 ± 2 . Determine the absorbances of the solutions at a wavelength of 340 nm. Add 0.05 mL of Solution B to each cell. Cover the cells to exclude oxygen. Mix by inversion, and allow to stand for 5 min at 22 ± 2
. Determine the absorbances of the solutions at a wavelength of 340 nm. Add 0.05 mL of Solution B to each cell. Cover the cells to exclude oxygen. Mix by inversion, and allow to stand for 5 min at 22 ± 2 . Determine the absorbances of the solutions at a wavelength of 340 nm.
. Determine the absorbances of the solutions at a wavelength of 340 nm.
Calculate the percentage of aldehydes, expressed as acetaldehyde, in the portion of Copovidone taken:
Result = 10 × (C/W) × [{(AU2  AU1)
AU1)  (AB2
(AB2  AB1)}/{(AS2
AB1)}/{(AS2  AS1)
AS1)  (AB2
(AB2  AB1)}]
AB1)}]
| C | = | = concentration of acetaldehyde in the Standard solution (mg/mL) |
| W | = | = weight, calculated on the dried basis, of Copovidone taken to prepare the Sample solution (g) |
| AU2 | = | = absorbance of the solution from the Sample solution, after the addition of Solution B |
| AU1 | = | = absorbance of the solution from the Sample solution, before the addition of Solution B |
| AB2 | = | = absorbance of the solution from the Blank solution, after the addition of Solution B |
| AB1 | = | = absorbance of the solution from the Blank solution, before the addition of Solution B |
| AS2 | = | = absorbance of the solution from the Standard solution, after the addition of Solution B |
| AS1 | = | = absorbance of the solution from the Standard solution, before the addition of Solution B |
Acceptance criteria:
NMT 0.05%
• Procedure 2: Limit of Hydrazine
Standard solution:
9 µg/mL of salicylaldazine and 10 mg/mL of salicylaldehyde, in toluene
Sample solution:
Transfer the equivalent of 2.5 g of dried Copovidone to a 50-mL centrifuge tube, add 25 mL of water, and mix to dissolve. Add 500 µL of a 50-mg/mL solution of salicylaldehyde in methanol, adjust the solution with 0.25 N sulfuric acid to a pH of about 2, swirl, and heat in a water bath at 60 for 15 min. Allow to cool, add 2.0 mL of toluene, insert a stopper in the tube, shake vigorously for 2 min, and centrifuge. Use the clear upper toluene layer.
for 15 min. Allow to cool, add 2.0 mL of toluene, insert a stopper in the tube, shake vigorously for 2 min, and centrifuge. Use the clear upper toluene layer.
Chromatographic system
Adsorbent:
0.25-mm layer of dimethylsilanized chromatographic silica gel mixture
Application volume:
10 µL
Developing solvent system:
Acetonitrile and water (17:3)
Analysis
Samples:
Standard solution and Sample solution
Proceed as directed in the chapter. Allow the spots to dry, and develop the chromatogram in the Developing solvent system until the solvent front has moved about three-fourths of the length of the plate. Locate the spots on the plate by examination under UV light at a wavelength of 365 nm: salicylaldazine appears as a fluorescent spot having an RF value of about 0.6–0.7, and the fluorescence of any salicylaldazine spot from the Sample solution is not more intense than that produced by the spot from the Standard solution.
Acceptance criteria:
NMT 1 ppm
• Procedure 3: Limit of Peroxides
Copovidone solution:
40 mg/mL of Copovidone in water, calculated on the dried basis
Sample solution:
Transfer 25.0 mL of Copovidone solution to a 50-mL beaker, and add 2 mL of titanium trichloride–sulfuric acid TS. Allow to stand for 30 min at room temperature.
Blank solution:
Transfer 25.0 mL of Copovidone solution to a 50-mL beaker, and add 2 mL of 13% sulfuric acid.
Spectrometric conditions
Mode:
UV-Vis
Analytical wavelength:
405 nm
Cell:
1 cm
Blank:
Blank solution
Analysis:
Determine the absorbance of the Sample solution.
Acceptance criteria:
The absorbance is NMT 0.35 (corresponding to NMT 0.04%, expressed as hydrogen peroxide.)
• Procedure 4: Limit of Monomers
Sample solution:
Dissolve the equivalent of 5.0 g of dried Copovidone in 20 mL of methanol, and slowly add 20.0 mL of iodobromide TS. Allow to stand for 30 min, protected from light, with repeated shaking. Add 5 mL of 100 mg/mL of potassium iodide solution.
Analysis:
Titrate the liberated iodine with 0.1 N sodium thiosulfate VS until the solution is yellow. Continue the titration dropwise until the solution is colorless, adding 3 mL of starch TS as the endpoint is approached. Perform a blank determination (see Titrimetry  541
541 , Residual Titrations).
, Residual Titrations).
Acceptance criteria:
The difference between the volumes of 0.1 N sodium thiosulfate consumed in the blank and the specimen titrations is NMT 0.9 mL, corresponding to NMT 0.1% of monomers calculated as vinylpyrrolidone.
SPECIFIC TESTS
• Loss on Drying  731
731 :
Dry a sample at 105
:
Dry a sample at 105 for 3 h: it loses NMT 5.0% of its weight.
for 3 h: it loses NMT 5.0% of its weight.
• Clarity and Color of Solution
Sample:
1.0 g
Analysis:
Dissolve the Sample in 10 mL of water.
Acceptance criteria:
The solution is clear or slightly opalescent and colorless to pale yellow or pale red.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers. No storage requirements specified.
• Labeling:
Label it to indicate its nominal K-value.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Robert H. Lafaver, M.S.
Scientific Liaison 1-301-816-8335 |
(EXC2010) Monographs - Excipients |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 1762
Pharmacopeial Forum: Volume No. 37(4)