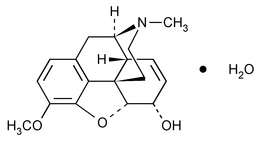
Codeine
(koe' deen).
Morphinan-6-ol, 7,8-didehydro-4,5-epoxy-3-methoxy-17-methyl-, monohydrate, (5
7,8-Didehydro-4,5
Anhydrous 299.37
» Codeine, dried at 80 for 4 hours, contains not less than 98.5 percent and not more than 100.5 percent of C18H21NO3.
for 4 hours, contains not less than 98.5 percent and not more than 100.5 percent of C18H21NO3.
Packaging and storage—
Preserve in tight, light-resistant containers.
Identification—
A:
Infrared Absorption  197K
197K —Proceed as directed with the codeine test specimen and the codeine standard specimen obtained from 50 mg of USP Codeine Sulfate RS dissolved in 15 mL of water, then rendered alkaline with 6 N ammonium hydroxide and extracted with several 10-mL portions of chloroform, followed by evaporation of the combined chloroform extracts on a steam bath to dryness, and drying at 80
—Proceed as directed with the codeine test specimen and the codeine standard specimen obtained from 50 mg of USP Codeine Sulfate RS dissolved in 15 mL of water, then rendered alkaline with 6 N ammonium hydroxide and extracted with several 10-mL portions of chloroform, followed by evaporation of the combined chloroform extracts on a steam bath to dryness, and drying at 80 for 4 hours.
for 4 hours.
B:
Ultraviolet Absorption  197U
197U —
—
Solution:
100 µg per mL.
Medium:
0.1 N sulfuric acid.
Absorptivity at 284 nm, calculated on the dried basis, is between 112.9% and 119.9% of that of USP Codeine Sulfate RS.
Melting range  741
741 —
When previously dried, it melts between 154
—
When previously dried, it melts between 154 and 158
and 158 , but the range between beginning and end of melting does not exceed 2
, but the range between beginning and end of melting does not exceed 2 .
.
Loss on drying  731
731 —
Dry it at 80
—
Dry it at 80 for 4 hours: it loses not more than 6.0% of its weight.
for 4 hours: it loses not more than 6.0% of its weight.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Readily carbonizable substances  271
271 —
Dissolve 10 mg in 5 mL of sulfuric acid: the solution has no more color than Matching Fluid S.
—
Dissolve 10 mg in 5 mL of sulfuric acid: the solution has no more color than Matching Fluid S.
Chromatographic purity—
Prepare a solution of it in dehydrated alcohol containing 40 mg per mL (Solution A). Dilute 2.0 mL of Solution A with dehydrated alcohol to 100 mL (Solution B). Dilute 1.0 mL of Solution A with dehydrated alcohol to 100 mL (Solution C). Apply separate 10-µL volumes of Solution A, Solution B, and Solution C to a suitable thin-layer chromatographic plate (see Chromatography  621
621 ) coated with a 0.25-mm layer of chromatographic silica gel. Allow the spots to dry, and develop the chromatogram in a solvent system consisting of a mixture of dehydrated alcohol, cyclohexane, and ammonium hydroxide (72:30:6) until the solvent front has moved three-fourths of the length of the plate. Remove the plate from the developing chamber, and allow the solvent to evaporate. Spray the plate with a reagent prepared by mixing 3 mL of chloroplatinic acid solution (1 in 10) with 97 mL of water, followed by the addition of 100 mL of potassium iodide solution (6 in 100), and examine the chromatogram: no spot obtained from Solution A, other than the principal spot and any spot observed at the origin, is more intense than the principal spot obtained from Solution B (2%); and not more than one such spot having an RF greater than that of the principal spot is more intense than the principal spot obtained from Solution C (1%).
) coated with a 0.25-mm layer of chromatographic silica gel. Allow the spots to dry, and develop the chromatogram in a solvent system consisting of a mixture of dehydrated alcohol, cyclohexane, and ammonium hydroxide (72:30:6) until the solvent front has moved three-fourths of the length of the plate. Remove the plate from the developing chamber, and allow the solvent to evaporate. Spray the plate with a reagent prepared by mixing 3 mL of chloroplatinic acid solution (1 in 10) with 97 mL of water, followed by the addition of 100 mL of potassium iodide solution (6 in 100), and examine the chromatogram: no spot obtained from Solution A, other than the principal spot and any spot observed at the origin, is more intense than the principal spot obtained from Solution B (2%); and not more than one such spot having an RF greater than that of the principal spot is more intense than the principal spot obtained from Solution C (1%).
Limit of morphine—
Dissolve about 50 mg of potassium ferricyanide in 10 mL of water, and add 1 drop of ferric chloride TS and 1 mL of a neutral or slightly acid solution of Codeine (1 in 100) prepared with the aid of sulfuric acid: no blue color is produced immediately.
Assay—
Dissolve about 400 mg of Codeine, previously dried and accurately weighed, by warming it in 30.0 mL of 0.1 N sulfuric acid VS. Cool, and add 10 mL of water. Add methyl red TS, and titrate the excess acid with 0.1 N sodium hydroxide VS. Perform a blank determination (see Residual Titrations under Titrimetry  541
541 ). Each mL of 0.1 N sulfuric acid is equivalent to 29.94 mg of C18H21NO3.
). Each mL of 0.1 N sulfuric acid is equivalent to 29.94 mg of C18H21NO3.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Clydewyn M. Anthony, Ph.D.
Senior Scientific Liaison 1-301-816-8139 |
(SM22010) Monographs - Small Molecules 2 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2758
Pharmacopeial Forum: Volume No. 34(5) Page 1151