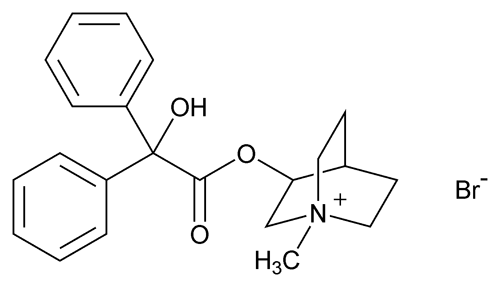
Clidinium Bromide
C22H26BrNO3 432.35
1-Azoniabicyclo[2.2.2]octane, 3-[(hydroxydiphenylacetyl)oxy]-1-methyl-, bromide, (±)-;
(±)-3-Hydroxy-1-methylquinuclidinium bromide benzilate

 [3485-62-9].
[3485-62-9].
(kli din' ee um broe' mide).
C22H26BrNO3 432.35
1-Azoniabicyclo[2.2.2]octane, 3-[(hydroxydiphenylacetyl)oxy]-1-methyl-, bromide, (±)-;
(±)-3-Hydroxy-1-methylquinuclidinium bromide benzilate
DEFINITION
Clidinium Bromide contains NLT 99.0% and NMT 100.5% of C22H26BrNO3, calculated on the dried basis.
IDENTIFICATION
• B.
The RF value of the principal spot of the Sample solution corresponds to that of the Standard solution, as obtained in the test for Organic Impurities.
• C. Bromide
Sample solution:
50 mg/mL
Analysis:
To 2 mL of the Sample solution add a few drops of 2 N nitric acid and 1 mL of silver nitrate TS.
Acceptance criteria:
A yellowish white precipitate is formed.
ASSAY
• Procedure
Sample:
1.2 g
Analysis:
Dissolve the Sample in 80 mL of glacial acetic acid, warming if necessary to effect solution. Cool, and add 15 mL of mercuric acetate TS. Titrate with 0.1 N perchloric acid in dioxane VS, determining the endpoint potentiometrically. Perform a blank determination (see Titrimetry  541
541 ). Each mL of 0.1 N perchloric acid is equivalent to 43.24 mg of C22H26BrNO3.
). Each mL of 0.1 N perchloric acid is equivalent to 43.24 mg of C22H26BrNO3.
Acceptance criteria:
99.0%–100.5% on the dried basis
IMPURITIES
• Residue on Ignition  281
281 :
NMT 0.1%
:
NMT 0.1%
• Organic Impurities
Standard solution:
100 mg/mL of USP Clidinium Bromide RS in 0.1 N methanolic hydrochloric acid
Sample solution:
100 mg/mL of Clidinium Bromide in 0.1 N methanolic hydrochloric acid
Reference solution:
Dissolve 100 mg of USP Clidinium Bromide RS in 1.0 mL of 0.1 N methanolic hydrochloric acid, and add 20 µL of a solution of 25.0 mg of USP Clidinium Bromide Related Compound A RS in 1.0 mL of 0.1 N methanolic hydrochloric acid.
Chromatographic system
Adsorbent:
0.25-mm layer of chromatographic silica gel mixture
Application volume:
20 µL
Developing solvent system:
Acetone, methanol, hydrochloric acid, and water (70:20:5:5)
Spray reagent:
Dissolve 850 mg of bismuth subnitrate in a mixture of 10 mL of glacial acetic acid and 40 mL of water. In a separate container, dissolve 20 g of potassium iodide in 50 mL of water. Mix the two solutions, and dilute with dilute sulfuric acid (1 in 10) to 500 mL. Add 7.5 g ± 2.5 g of iodine, and mix until the solution is complete.
Chromatographic plates:
Predevelop suitable thin-layer chromatographic plates by placing in a chromatographic chamber saturated with the Developing solvent system, and allow the Developing solvent system to move about 15 cm. Remove the plates from the chamber, dry at 105 for 15 min, and cool.
for 15 min, and cool.
Analysis 1 (3-quinuclidinyl benzilate):
Apply the Standard solution and the Sample solution to a Chromatographic plate. Place the plate in an unsaturated chromatographic chamber containing freshly prepared Developing solvent system, and allow the solvent front to move 10 cm. Remove the plate, dry at 105 for 10 min, cool, and spray with potassium iodoplatinate TS.
for 10 min, cool, and spray with potassium iodoplatinate TS.
Acceptance criteria 1:
The Sample solution shows no spot at an RF value (about 0.8) corresponding to that of 3-quinuclidinyl benzilate.
Analysis 2 (limit of clidinium bromide related compound A):
Apply the Sample solution and Reference solution to a second Chromatographic plate. Place the plate in an unsaturated chromatographic chamber containing freshly prepared Developing solvent system, and allow the solvent front to move 15 cm. Remove the plate, dry at 105 for 10 min, cool, and spray with the Spray reagent.
for 10 min, cool, and spray with the Spray reagent.
Acceptance criteria 2:
Any spot from the Sample solution at an RF value of about 0.4 is not greater in size or intensity than the minor spot of the Reference solution: NMT 0.5% of clidinium bromide related compound A is found.
SPECIFIC TESTS
• Loss on Drying  731
731 :
Dry a sample at 105
:
Dry a sample at 105 for 3 h: it loses NMT 0.5% of its weight.
for 3 h: it loses NMT 0.5% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight, light-resistant containers.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Elena Gonikberg, Ph.D.
Principal Scientific Liaison 1-301-816-8251 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2701
Pharmacopeial Forum: Volume No. 27(4) Page 2720