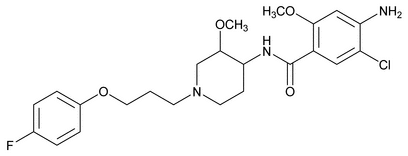
Cisapride
(sis' a pride).
Benzamide, 4-amino-5-chloro-N-[1-[3-(4-fluorophenoxy)propyl]-3-methoxy-4-piperidinyl]-2-methoxy-, cis-
cis-4-Amino-5-chloro-N-[1-[3-(p-fluorophenoxy)propyl]-3-methoxy-4-piperidyl]-o-anisamide
Monohydrate 484.0
» Cisapride contains not less than 99.0 percent and not more than 101.0 percent of C23H29ClFN3O4, calculated on the anhydrous basis.
Packaging and storage—
Preserve in well-closed, light-resistant containers, and store at room temperature.
Completeness of solution  641
641 —
A solution, 10 mg per mL in methylene chloride, meets the requirements.
—
A solution, 10 mg per mL in methylene chloride, meets the requirements.
Identification,
Infrared Absorption  197K
197K .
.
Specific rotation  781S
781S :
between
:
between  10
10 and +10
and +10 , measured at 20
, measured at 20 .
.
Test solution:
10 mg per mL, in methylene chloride.
Water, Method I  921
921 :
between 3.4% and 4.0%.
:
between 3.4% and 4.0%.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Chromatographic purity—
Solution A—
Prepare a 20 g per L solution of tetrabutylammonium hydrogen sulfate in water.
Solution B—
Use methanol.
Mobile phase—
Use variable mixtures of Solution A and Solution B as directed for Chromatographic system. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Blank solution—
Use methanol.
System suitability solution—
Prepare a solution of USP Cisapride RS and USP Haloperidol RS in methanol containing about 0.05 mg per mL and 0.4 mg per mL, respectively.
Test solution 1—
Dissolve an accurately weighed quantity of Cisapride, in methanol to obtain a solution having a known concentration of about 10 mg per mL.
Test solution 2—
Dilute quantitatively and stepwise Test solution 1 in methanol to obtain a solution having a known concentration of about 0.05 mg per mL.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 275-nm detector and a 4.0-mm × 10-cm column that contains 3-µm base-deactivated packing L1. The flow rate is about 1.2 mL per minute. The chromatograph is programmed as follows.
)—
The liquid chromatograph is equipped with a 275-nm detector and a 4.0-mm × 10-cm column that contains 3-µm base-deactivated packing L1. The flow rate is about 1.2 mL per minute. The chromatograph is programmed as follows.
Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the order of elution is cisapride followed by haloperidol, the resolution, R, between these two peaks is not less than 2.5; and the relative standard deviation for replicate injections is not more than 2.0% for the cisapride peak.
| Time (minutes) |
Solution A
(%) |
Solution B
(%) |
Elution |
|---|---|---|---|
| 0–20 | 80®55 | 20®45 | linear gradient |
| 20–21 | 55®5 | 45®95 | linear gradient |
| 21–25 | 5 | 95 | isocratic |
| 25–26 | 5®80 | 95®20 | return to initial conditions |
| 26–30 | 80 | 20 | re-equilibration |
Procedure—
Inject a volume (about 10 µL) of the Blank solution, Test solution 1, and Test solution 2 into the chromatograph, record the chromatograms, and measure the peak areas. Calculate the percentage of cisapride impurities in the portion of Cisapride taken by the formula:
100(CS / Ci)(ri / rS)
in which CS and Ci are the concentration of cisapride, in mg per mL, of Test solution 2 and Test solution 1, respectively; ri is the individual peak response of cisapride inpurities in Test solution 1; and rS is cisapride peak area in Test solution 2: not more than 0.5% of any cisapride impurity is found, and not more than 1.0% of total impurities is found. Disregard any peak also found in the Blank solution and any peak with an area less than 0.1 times the area of the principal peak in the Test solution 2 chromatogram.
Assay—
Dissolve about 0.350 g of Cisapride, accurately weighed, in 70 mL of a mixture of methyl ethyl ketone and acetic acid (7:1). Titrate with 0.1 N perchloric acid VS, determining the endpoint potentiometrically. Perform a blank determination, and make any necessary correction (see Titrimetry  541
541 ). Each mL of 0.1 N perchloric acid is equivalent to 46.60 mg of C23H29ClFN3O4.
). Each mL of 0.1 N perchloric acid is equivalent to 46.60 mg of C23H29ClFN3O4.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Morgan Puderbaugh, B.S.
Associate Scientific Liaison 1-301-998-6833 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2677
Pharmacopeial Forum: Volume No. 34(2) Page 253