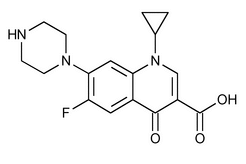
Ciprofloxacin
C17H18FN3O3 331.34
3-Quinolinecarboxylic acid, 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-;
1-Cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid

 [85721-33-1].
[85721-33-1].
(sip'' roe flox' a sin).
C17H18FN3O3 331.34
3-Quinolinecarboxylic acid, 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-;
1-Cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid
DEFINITION
Ciprofloxacin contains NLT 98.0% and NMT 102.0% of C17H18FN3O3, calculated on the dried basis.
IDENTIFICATION
• A. Infrared Absorption:
The IR absorption spectrum of a potassium bromide dispersion of it exhibits maxima at the same wavelengths as that of a similar preparation of USP Ciprofloxacin RS.
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
Solution A:
0.025 M phosphoric acid. Adjust with triethylamine to a pH of 3.0 ± 0.1.
Mobile phase:
Acetonitrile and Solution A (13:87)
Standard solution:
Transfer 12.5 mg of USP Ciprofloxacin RS to a 25-mL volumetric flask. Add 0.1 mL of 7% phosphoric acid, and dilute with Mobile phase to volume.
System suitability stock solution:
0.025 mg/mL of USP Ciprofloxacin Ethylenediamine Analog RS in Mobile phase
System suitability solution:
Transfer 1.0 mL of the System suitability stock solution to a 10-mL volumetric flask, and dilute with the Standard solution to volume.
Sample solution:
Transfer 25 mg of Ciprofloxacin to a 50-mL volumetric flask. Add 0.2 mL of 7% phosphoric acid, and dilute with Mobile phase to volume.
Chromatographic system
Mode:
LC
Detector:
UV 278 nm
Column:
4.6-mm × 25-cm; packing L1
Column temperature:
30 ± 1
Flow rate:
1.5 mL/min
Injection size:
10 µL
System suitability
Samples:
Standard solution and System suitability solution
[Note—The relative retention times for ciprofloxacin ethylenediamine analog and ciprofloxacin are about 0.7 and 1.0, respectively. ]
Suitability requirements
Resolution:
NLT 6 between ciprofloxacin ethylenediamine analog and ciprofloxacin, System suitability solution
Column efficiency:
NLT 2500 theoretical plates from the ciprofloxacin peak, Standard solution
Tailing factor:
NMT 2.5 for the ciprofloxacin peak, Standard solution
Relative standard deviation:
NMT 1.5%, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C17H18FN3O3 in the portion of Ciprofloxacin taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak area from the Sample solution |
| rS | = | = peak area from the Standard solution |
| CS | = | = concentration of USP Ciprofloxacin RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Ciprofloxacin in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–102.0% on the dried basis
IMPURITIES
Inorganic Impurities
• Residue on Ignition  281
281 :
NMT 0.1%, except that where it is intended for use in preparing Ciprofloxacin for Oral Suspension, it is NMT 0.2%.
:
NMT 0.1%, except that where it is intended for use in preparing Ciprofloxacin for Oral Suspension, it is NMT 0.2%.
• Chloride
Standard solution:
8.2 µg/mL of sodium chloride (5µg/mL of chloride)
Sample solution:
Add 30.0 mL of water to 0.5 g of Ciprofloxacin, shake for 5 min, and pass through chloride-free filter paper. Use the filtrate as the Sample solution.
Analysis
Samples:
Standard solution and Sample solution
Transfer 15.0 mL of the Sample solution to a 50-mL color-comparison tube. Transfer 10.0 mL of the Standard solution to a second matched 50-mL color-comparison tube, add 5.0 mL of water, and mix. To each tube add 1 mL of 2 N nitric acid, mix, add 1 mL of silver nitrate TS, and mix.
Acceptance criteria:
The turbidity exhibited by the Sample solution does not exceed that of the Standard solution (0.02%).
• Sulfate
Standard solution:
18.1 µg/mL of potassium sulfate in 30% alcohol (10 µg/mL of sulfate)
Sample solution:
Dissolve 0.5 g of Ciprofloxacin in 5.0 mL of 2 N acetic acid and 15.0 mL of water.
Analysis
Samples:
Standard solution and Sample solution
To each of two 50-mL matched color-comparison tubes transfer 1.50 mL of the Standard solution. To each tube add, successively and with continuous shaking, 1.0 mL of 250 mg/mL barium chloride solution, and allow to stand for 1 min. To one of the tubes transfer 15.0 mL of the Standard solution and 0.5 mL of 30% acetic acid, and mix. To the second tube add 15.0 mL of the Sample solution and 0.5 mL of 30% acetic acid, and mix.
Acceptance criteria:
The turbidity exhibited in the tube containing the Sample solution does not exceed that of the tube containing the Standard solution (0.04%).
• Heavy Metals, Method II  231
231 :
NMT 20 ppm
:
NMT 20 ppm
Organic Impurities
• Procedure 1: Limit of Fluoroquinolonic Acid
Standard stock solution:
Transfer 5.0 mg of USP Fluoroquinolonic Acid RS to a 50-mL volumetric flask containing 0.05 mL of 6 N ammonium hydroxide, and dilute with water to volume.
Standard solution:
Dilute 2.0 mL of the Standard stock solution with water to 10.0 mL.
Sample solution:
10.0 mg/mL of Ciprofloxacin in 0.1 N acetic acid
Developing solvent system:
Methylene chloride, methanol, acetonitrile, and ammonium hydroxide (4:4:1:2)
Chromatographic system
Mode:
TLC
Adsorbent:
0.25-mm layer of silica gel mixture
Application volume:
5 µL
Analysis
Samples:
Standard solution and Sample solution
Place the plate in a suitable chamber in which is placed a beaker containing 50 mL of ammonium hydroxide. After 15 min, transfer the plate to a suitable chromatographic chamber, and develop the chromatogram in the Developing solvent system until the solvent front has moved three-fourths of the length of the plate. Remove the plate from the chamber, mark the solvent front, and allow the plate to air-dry for about 15 min. Examine the plate under short-wavelength UV light.
Acceptance criteria:
Any spot from the Sample solution, at an RF value corresponding to the principal spot from the Standard solution, is not greater in size or intensity than the principal spot from the Standard solution (0.2%).
• Procedure 2
Solution A, Mobile phase, System suitability stock solution, System suitability solution, Standard solution, Sample solution, Chromatographic system, and System suitability:
Proceed as directed in the Assay.
Analysis
Sample:
Sample solution
Calculate the percentage of each impurity in the portion of Ciprofloxacin taken:
Result = (rU/rT) × 100
| rU | = | = response of each impurity peak |
| rT | = | = sum of the responses of all the peaks |
Acceptance criteria
Ciprofloxacin ethylenediamine analog or any other individual impurity peak:
NMT 0.2%
Total impurities:
NMT 0.5%
SPECIFIC TESTS
• Clarity of Solution:
Dissolve 0.25 g in 10 mL of 0.1 N hydrochloric acid: a clear to slightly opalescent solution is obtained.
• Microbial Enumeration Tests  61
61 and Tests for Specified Microorganisms
and Tests for Specified Microorganisms  62
62 :
Where it is intended for use in preparing Ciprofloxacin for Oral Suspension, the total microbial count does not exceed 1000 cfu/g, and the total combined molds and yeasts count does not exceed 100 cfu/g. It also meets the requirement for absence of Salmonella species and Escherichia coli.
:
Where it is intended for use in preparing Ciprofloxacin for Oral Suspension, the total microbial count does not exceed 1000 cfu/g, and the total combined molds and yeasts count does not exceed 100 cfu/g. It also meets the requirement for absence of Salmonella species and Escherichia coli.
• Loss on Drying  731
731 :
Dry a sample in a vacuum at 120
:
Dry a sample in a vacuum at 120 for 6 h: it loses NMT 1.0% of its weight, except that where it is labeled as intended for use in preparing Ciprofloxacin for Oral Suspension, it loses between 10% and 20% of its weight.
for 6 h: it loses NMT 1.0% of its weight, except that where it is labeled as intended for use in preparing Ciprofloxacin for Oral Suspension, it loses between 10% and 20% of its weight.
• Sterility Tests  71
71 :
Where the label states that it is sterile, it meets the requirements for Test for Sterility of the Product to Be Examined, Membrane Filtration.
:
Where the label states that it is sterile, it meets the requirements for Test for Sterility of the Product to Be Examined, Membrane Filtration.
• Bacterial Endotoxins Test  85
85 :
Where the label states that it is sterile or where the label states that Ciprofloxacin must be subjected to further processing during the preparation of injectable dosage forms, it contains NMT 0.50 USP Endotoxin Unit/mg of ciprofloxacin.
:
Where the label states that it is sterile or where the label states that Ciprofloxacin must be subjected to further processing during the preparation of injectable dosage forms, it contains NMT 0.50 USP Endotoxin Unit/mg of ciprofloxacin.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight, light-resistant containers. Store at 25 , excursion permitted between 15
, excursion permitted between 15 and 30
and 30 , and avoid excessive heat.
, and avoid excessive heat.
• Labeling:
Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms. Where it is intended for use in preparing Ciprofloxacin for Oral Suspension, it is so labeled.
• USP Reference Standards  11
11
USP Ciprofloxacin Ethylenediamine Analog RS 
1-Cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-[(2-aminoethyl)amino]-3-quinolinecarboxylic acid hydrochloride.
C15H16FN3O3·HCl 341.77

1-Cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-[(2-aminoethyl)amino]-3-quinolinecarboxylic acid hydrochloride.
C15H16FN3O3·HCl 341.77
USP Endotoxin RS
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Behnam Davani, Ph.D., M.B.A.
Senior Scientific Liaison 1-301-816-8394 |
(SM12010) Monographs - Small Molecules 1 |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2669
Pharmacopeial Forum: Volume No. 35(4) Page 837