Chondroitin Sulfate Sodium
Chondroitin, hydrogen sulfate, sodium salt

 [9082-07-9].
[9082-07-9].
Chondroitin, hydrogen sulfate, sodium salt
DEFINITION
Chondroitin Sulfate Sodium is the sodium salt of the sulfated linear glycosaminoglycan obtained from bovine, porcine, or avian cartilages of healthy and domestic animals used for food by humans. Chondroitin Sulfate Sodium consists mostly of the sodium salt of the sulfate ester of N-acetylchondrosamine (2-acetamido-2-deoxy-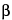 -d-galactopyranose) and d-glucuronic acid copolymer. These hexoses are alternately linked
-d-galactopyranose) and d-glucuronic acid copolymer. These hexoses are alternately linked 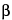 -1,4 and
-1,4 and 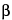 -1,3 in the polymer. Chondrosamine moieties in the prevalent glycosaminoglycan are monosulfated primarily on position 4 and less so on position 6. It contains NLT 90.0% and NMT 105.0% of chondroitin sulfate sodium, calculated on the dried basis.
-1,3 in the polymer. Chondrosamine moieties in the prevalent glycosaminoglycan are monosulfated primarily on position 4 and less so on position 6. It contains NLT 90.0% and NMT 105.0% of chondroitin sulfate sodium, calculated on the dried basis.
[Note—Chondroitin Sulfate Sodium is extremely hygroscopic once dried. Avoid exposure to the atmosphere, and weigh promptly. ]
IDENTIFICATION
• B. Identification Tests—General, Sodium  191
191 :
Meets the requirements
:
Meets the requirements
Sample solution:
0.5 g in 10 mL of water
COMPOSITION
• Content of Chondroitin Sulfate Sodium
Standard solutions:
1.5, 1.0, and 0.5 mg/mL of USP Chondroitin Sulfate Sodium RS in water
Sample solution:
Transfer 100 mg of dried Chondroitin Sulfate Sodium into a 100-mL volumetric flask, dissolve in 30 mL of water, and dilute with water to volume.
Diluent:
Weigh about 297 mg of monobasic potassium phosphate, 492 mg of dibasic potassium phosphate, and 250 mg of polysorbate 80, and transfer into a 1-L beaker. Dissolve in 900 mL of water, and adjust with potassium hydroxide or phosphoric acid to a pH of 7.0 ± 0.2. Dilute with water to 1 L, and mix thoroughly.
Titrimetric system
(See Titrimetry  541
541 .)
.)
Mode:
Photometric titration
Titrant:
1 mg/mL of cetylpyridinium chloride in water. Degas before use.
Endpoint detection:
Turbidimetric with a photoelectric probe
Analysis:
Transfer 5.0 mL of each Standard solution and the Sample solution to separate titration vessels, and add 25 mL of Diluent to each. Stir until a steady reading is obtained with a phototrode either at 420, 550, or 660 nm. Set the instrument to zero in absorbance mode. Titrate with Titrant using the phototrode to determine the endpoint turbidimetrically. From a linear regression equation, calculated using the volumes of Titrant consumed versus concentrations of the Standard solutions, determine the concentration of chondroitin sulfate sodium in the Sample solution.
Calculate the percentage of chondroitin sulfate sodium in the portion Chondroitin Sulfate Sodium taken:
Result = (C/CU) × 100
| C | = | = concentration of chondroitin sulfate sodium in the aliquot of the Sample solution, obtained from the regression equation (mg/mL) |
| CU | = | = concentration of Chondroitin Sulfate Sodium in the Sample solution (mg/mL) |
Acceptance criteria:
90.0%–105.0% on the dried basis
IMPURITIES
• Residue on Ignition  281
281 :
20.0%–30.0% on the dried basis
:
20.0%–30.0% on the dried basis
• Chloride and Sulfate, Chloride  221
221 :
NMT 0.50%; a 0.10-g portion shows no more chloride than corresponds to 0.7 mL of 0.020 N hydrochloric acid.
:
NMT 0.50%; a 0.10-g portion shows no more chloride than corresponds to 0.7 mL of 0.020 N hydrochloric acid.
• Chloride and Sulfate, Sulfate  221
221
Sample solution:
Dissolve 200 mg in 40 mL of water. Add 10 mL of a solution of cetylpyridinium chloride having a concentration of 30 mg/mL, and pass through a filter. Use a 25-mL portion of the filtrate.
Acceptance criteria:
NMT 0.24%; the Sample solution shows no more sulfate than corresponds to 0.25 mL of 0.020 N sulfuric acid.
• Electrophoretic Purity
(See Electrophoresis  726
726 .)
.)
Barium acetate buffer:
Dissolve 25.24 g of barium acetate in 900 mL of water. Adjust with acetic acid to a pH of 5.0, and dilute with water to 1000 mL.
Staining reagent:
Dissolve 1 g of toluidine blue in 1000 mL of 0.1 M acetic acid.
Standard solution A:
30 mg/mL of USP Chondroitin Sulfate Sodium RS in water
Standard solution B:
Dilute 1 mL of Standard solution A with water to 50 mL.
Sample solution:
30 mg/mL of Chondroitin Sulfate Sodium in water
Analysis:
Fill the chambers of an electrophoresis apparatus suitable for separations on cellulose acetate membranes1 (a small submarine gel chamber or one dedicated to membrane media) with Barium acetate buffer. Soak a cellulose acetate membrane, 5–6 cm × 12–14 cm, in Barium acetate buffer for 10 min, or until evenly wetted, then blot dry between two sheets of absorbent paper. Using an applicator2 suitable for electrophoresis, apply equal volumes (0.5 µL) of the Sample solution, Standard solution A, and Standard solution B to the brighter side of the membrane held in position in an appropriate applicator stand or on a separating bridge in the chamber. Ensure that both ends of the membrane are dipped at least 0.5–1.0 cm deep into the buffer chambers. Apply a constant 60 volts (6 mA at the start) for 2 h. [Note—Perform the application of solutions and voltage within 5 min because further drying of the blotted paper reduces sensitivity. ]
Place the membrane in a plastic staining tray, and with the application side down, float or gently immerse in Staining reagent for 5 min. Then stir the solution gently for 1 min. Remove the membrane, and destain in 5% acetic acid until the background clears. Compare the bands.
[Note—Document the results by taking a picture within 15 min of completion of destaining. ]
Acceptance criteria:
The electropherogram from the Sample solution exhibits a major band that is identical in position to the band from Standard solution A. The band from Standard solution B is clearly visible at a mobility similar to the band from Standard solution A. Any secondary band in the electropherogram of the Sample solution is not more intense than the band from Standard solution B. NMT 2% of any individual impurity is found. [Note—Document the results by taking a picture within 15 min of completion of destaining. ]
• Limit of Protein
Solution A:
20 mg/mL of sodium tartrate dihydrate
Solution B:
10 mg/mL of cupric sulfate
Solution C:
20 mg/mL of anhydrous sodium carbonate in 0.1 M sodium hydroxide
Dilute Folin-Ciocalteu reagent:
Dilute Folin-Ciocalteu phenol TS with water (1:5). Prepare immediately before use.
Alkaline cupric tartaric reagent:
Mix 1 mL each of Solution A and Solution B, and to the mixture slowly add 100 mL of Solution C with stirring. Use within 24 h, and discard afterward.
Standard solution:
36 µg/mL of bovine serum albumin certified standard in water
Sample solution:
Transfer a portion of Chondroitin Sulfate Sodium, equivalent to 60 mg of the dried substance, to a 100-mL volumetric flask, and dissolve in and dilute with water to volume.
Instrumental conditions
Analytical wavelength:
750 nm
Blank:
Water
Analysis
Samples:
Standard solution, Sample solution, and Blank
Add 2.0 mL of freshly prepared Alkaline cupric tartaric reagent to test tubes containing 2.0 mL of the Standard solution, 2.0 mL of the Sample solution, or 2.0 mL of the Blank. After 10 min, add 1.0 mL of Dilute Folin-Ciocalteu reagent to each test tube, and mix immediately and vigorously. After 30 min, measure the absorbance of the Standard solution and Sample solution against the Blank.
Acceptance criteria:
NMT 6.0% on the dried basis; the absorbance of the Sample solution is NMT the absorbance of the Standard solution.
CONTAMINANTS
• Microbial Enumeration Tests  2021
2021 :
The total bacterial count does not exceed 1000 cfu/g, and the total combined molds and yeasts count does not exceed 100 cfu/g.
:
The total bacterial count does not exceed 1000 cfu/g, and the total combined molds and yeasts count does not exceed 100 cfu/g.
• Microbial Procedures for Absence of Specified Microorganisms  2022
2022 :
It meets the requirements of the tests for absence of Salmonella species, and Escherichia coli.
:
It meets the requirements of the tests for absence of Salmonella species, and Escherichia coli.
• Heavy Metals, Method II  231
231 :
NMT 20 ppm
:
NMT 20 ppm
SPECIFIC TESTS
• Clarity and Color of Solution
Sample solution:
Transfer 2.5 g of Chondroitin Sulfate Sodium to a 50-mL volumetric flask. Dissolve in and dilute with carbon dioxide-free water to volume, and examine immediately.
Instrumental conditions
Analytical wavelength:
420 nm
Cell:
1 cm
Blank:
Carbon dioxide-free water
Analysis:
Measure the absorbance of the Sample solution.
Acceptance criteria:
Its absorbance is NMT 0.35.
• pH  791
791 :
5.5–7.5, in a solution (1 in 100)
:
5.5–7.5, in a solution (1 in 100)
• Loss on Drying  731
731 :
Dry a sample at 105
:
Dry a sample at 105 for 4 h: it loses NMT 10.0% of its weight. [Note—Chondroitin Sulfate Sodium is extremely hygroscopic once dried. Avoid exposure to the atmosphere, and weigh promptly. ]
for 4 h: it loses NMT 10.0% of its weight. [Note—Chondroitin Sulfate Sodium is extremely hygroscopic once dried. Avoid exposure to the atmosphere, and weigh promptly. ]
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers.
• Labeling:
Label it to state the source(s) from which the article was derived, whether bovine, porcine, avian, or a mixture of any of them.
1
Suitable cellulose acetate membranes for electrophoresis are available from Malta Chemetron SRL, Milano, Italy (www.maltachemetron.com); Fluka Chemical Corp., Milwaukee, WI; and DiaSys Corp., Waterbury, CT (www.diasys.com).
2
Suitable applicators are available from DiaSys Corp., Waterbury, CT (www.diasys.com) and Helena Laboratories, Beaumont, TX (www.helena.com).
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Huy T. Dinh, M.S.
Scientific Liaison 1-301-816-8594 |
(DS2010) Monographs - Dietary Supplements |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 1248
Pharmacopeial Forum: Volume No. 30(6) Page 2068
