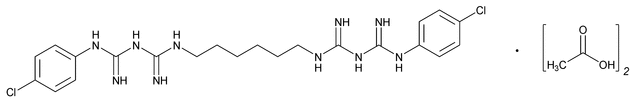
Chlorhexidine Acetate
C22H30Cl2N10·2C2H4O2 625.55
2,4,11,13-Tetraazatetradecanediimidamide,N,N¢¢-bis(4-chlorophenyl)-3,12-diimino-, diacetate;
1,1¢-Hexamethylenebis[5-(p-chlorophenyl)biguanide] diacetate

 [56-95-1].
[56-95-1].
(klor hex' i deen as' e tate).
C22H30Cl2N10·2C2H4O2 625.55
2,4,11,13-Tetraazatetradecanediimidamide,N,N¢¢-bis(4-chlorophenyl)-3,12-diimino-, diacetate;
1,1¢-Hexamethylenebis[5-(p-chlorophenyl)biguanide] diacetate
DEFINITION
Chlorhexidine Acetate contains NLT 98.0% and NMT 101.0% of C22H30Cl2N10·2C2H4O2, calculated on the dried basis.
IDENTIFICATION
ASSAY
• Procedure
Solution A:
27.6 g of monobasic sodium phosphate and 10 mL of triethylamine in 1.5 L of water. Adjust with phosphoric acid to a pH of 3.0, and dilute with water to 2000 mL. Prepare a mixture of acetonitrile and this solution (3:7).
Solution B:
Acetonitrile
Mobile phase:
See the gradient table below.
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 100 | 0 |
| 9 | 100 | 0 |
| 10 | 45 | 55 |
| 15 | 45 | 55 |
| 16 | 100 | 0 |
| 21 | 100 | 0 |
Standard solution:
50 µg/mL of USP Chlorhexidine Acetate RS in Solution A
System suitability solution:
50 µg/mL of USP Chlorhexidine Acetate RS and 1 µg/mL of USP p-Chloroaniline RS in Solution A
Sample solution:
50 µg/mL of Chlorhexidine Acetate in Solution A
Chromatographic system
Mode:
LC
Detector:
UV 239 nm
Column:
4.6-mm × 25-cm; base-deactivated 5-µm packing L1
Column temperature:
40
Flow rate:
1.5 mL/min
Injection size:
50 µL
System suitability
Sample:
System suitability solution
[Note—The approximate relative retention times for chlorhexidine and p-chloroaniline are about 1.0 and 1.3, respectively. ]
Suitability requirements
Resolution:
NLT 3 between chlorhexidine and p-chloroaniline
Relative standard deviation:
NMT 2.0% for chlorhexidine and NMT 5.0% for p-chloroaniline
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C22H30Cl2N10·2C2H4O2 in the portion of Chlorhexidine Acetate taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of chlorhexidine from the Sample solution |
| rS | = | = peak response of chlorhexidine from the Standard solution |
| CS | = | = concentration of USP Chlorhexidine Acetate RS in the Standard solution (µg/mL) |
| CU | = | = concentration of Chlorhexidine Acetate in the Sample solution (µg/mL) |
Acceptance criteria:
98.0%–101.0% on the dried basis
IMPURITIES
Inorganic Impurities
• Residue on Ignition  281
281 :
NMT 0.15%
:
NMT 0.15%
Organic Impurities
• Procedure
Solution A and Solution B:
Proceed as directed in the Assay.
Diluent:
27.6 g of monobasic sodium phosphate in 1.5 L of water. Adjust with phosphoric acid to a pH of 3.0, and dilute with water to 2000 mL.
Mobile phase:
See the gradient table below.
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 100 | 0 |
| 15 | 100 | 0 |
| 16 | 45 | 55 |
| 21 | 45 | 55 |
| 22 | 100 | 0 |
| 27 | 100 | 0 |
Sample solution:
2 mg/mL of Chlorhexidine Acetate in Solution A
Standard solution A:
0.06 mg/mL of Chlorhexidine Acetate in Solution A, from Sample solution
Standard solution B:
1.2 µg/mL of Chlorhexidine Acetate in Solution A, from Standard solution A
System suitability solution:
10 mg of USP Chlorhexidine Related Compounds RS to a 10-mL volumetric flask. Dissolve in 2 mL of acetonitrile, and dilute with Diluent to volume.
Chromatographic system:
Proceed as directed in the Assay. [Note—Injection size is 20 µL. ]
System suitability
Sample:
System suitability solution
[Note—The relative retention times for the main related compound peak and chlorhexidine are 0.6 and 1.0, respectively. ]
Suitability requirements
Resolution:
The two peaks between the main related compound peak and the chlorhexidine peak should be at least partially resolved from each other and completely resolved from the chlorhexidine peak.
Analysis
Samples:
Standard solution A, Standard solution B, and Sample solution
Calculate the percentage of each impurity in the portion of Chlorhexidine Acetate taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of each impurity from the Sample solution |
| rS | = | = peak response of chlorhexidine from Standard solution A |
| CS | = | = concentration of chlorhexidine acetate in Standard solution A (µg/mL) |
| CU | = | = concentration of Chlorhexidine Acetate in the Sample solution (µg/mL) |
Acceptance criteria
Total impurities:
NMT 3.0%. [Note—Disregard any peak less than the area of the chlorhexidine peak as obtained from Standard solution B. ]
SPECIFIC TESTS
• Limit of p-Chloroaniline
Solution A, Solution B, Mobile phase, System suitability solution, and Chromatographic system:
Proceed as directed in the Assay.
Standard solution:
1.0 µg/mL of USP p-Chloroaniline RS in Solution A
Sample solution:
2.0 mg/mL of Chlorhexidine Acetate in Solution A
Analysis
Samples:
Standard solution and Sample solution
Acceptance criteria
Total impurities:
The p-chloroaniline peak area of the Sample solution is NMT the p-chloroaniline peak area in the Standard solution (NMT 500 ppm).
• Loss on Drying  731
731 :
Dry a sample at 105
:
Dry a sample at 105 to constant weight: it loses NMT 3.5% of its weight.
to constant weight: it loses NMT 3.5% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed, light-resistant containers.
• USP Reference Standards  11
11
USP Chlorhexidine Acetate RS
USP Chlorhexidine Related Compounds RS
USP p-Chloroaniline RS
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Morgan Puderbaugh, B.S.
Associate Scientific Liaison 1-301-998-6833 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2621
Pharmacopeial Forum: Volume No. 36(1) Page 85