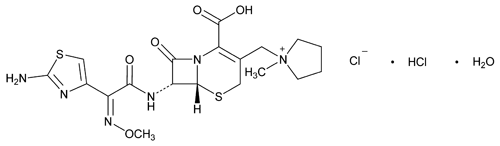
Cefepime Hydrochloride
(sef' e peem hye'' droe klor' ide).
C19H25ClN6O5S2·HCl·H2O




 571.50
571.50
Pyrrolidinium, 1-[[7-[[(2-amino-4-thiazolyl)(methoxyimino)acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-1-methyl-, chloride, monohydrochloride, monohydrate, [6R-[6 ,7
,7 (Z)]]-.
(Z)]]-.
1-[[(6R,7R)-7-[2-(2-Amino-4-thiazolyl)glyoxylamido]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-1-methylpyrrolidinium chloride, 72-(Z)-(O-methyloxime), monohydrochloride, monohydrate

 [123171-59-5].
[123171-59-5].
Pyrrolidinium, 1-[[7-[[(2-amino-4-thiazolyl)(methoxyimino)acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-1-methyl-, chloride, monohydrochloride, monohydrate, [6R-[6
1-[[(6R,7R)-7-[2-(2-Amino-4-thiazolyl)glyoxylamido]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-1-methylpyrrolidinium chloride, 72-(Z)-(O-methyloxime), monohydrochloride, monohydrate
» Cefepime Hydrochloride contains the equivalent of not less than 825 µg and not more than 911 µg of cefepime (C19H24N6O5S2) per mg, calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight, light-resistant containers, and store at controlled room temperature.
Labeling—
Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
USP Reference standards  11
11 —
—
USP Cefepime Hydrochloride System Suitability RS 

This is a mixture of cefepime hydrochloride related compound A ([6R-[6 ,7
,7 (E)]]-1-[[7-[[(2-amino-4-thiazolyl) (methoxyimino)acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-1-methylpyrrolidinium chloride, monohydrochloride, monohydrate; (C19H25ClN6O5S2·HCl·H2O
(E)]]-1-[[7-[[(2-amino-4-thiazolyl) (methoxyimino)acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-1-methylpyrrolidinium chloride, monohydrochloride, monohydrate; (C19H25ClN6O5S2·HCl·H2O  571.50); cefepime related compound B [6R-trans]-7-[[[2-[[(2-amino-4-thiazolyl)(methoxyimino)acetyl]amino]-4-thiazolyl](methoxyimino)acetyl]amino]-3-(1-methylpyrrolidinium-1-yl)methyl]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, inner salt; (C25H29N9O7S3
571.50); cefepime related compound B [6R-trans]-7-[[[2-[[(2-amino-4-thiazolyl)(methoxyimino)acetyl]amino]-4-thiazolyl](methoxyimino)acetyl]amino]-3-(1-methylpyrrolidinium-1-yl)methyl]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, inner salt; (C25H29N9O7S3  663.75); and cefepime hydrochloride.
663.75); and cefepime hydrochloride.
USP Endotoxin RS
Identification, Infrared Absorption  197M
197M .
.
Test specimen—
Proceed as directed in the chapter, but do not dry.
Crystallinity  695
695 :
meets the requirements.
:
meets the requirements.
Bacterial endotoxins  85
85 —
Where the label states that Cefepime Hydrochloride is sterile or that it must be subjected to further processing during the preparation of injectable dosage forms, it contains not more than 0.04 USP Endotoxin Unit per mg of cefepime hydrochloride.
—
Where the label states that Cefepime Hydrochloride is sterile or that it must be subjected to further processing during the preparation of injectable dosage forms, it contains not more than 0.04 USP Endotoxin Unit per mg of cefepime hydrochloride.
Water, Method I  921
921 :
between 3.0% and 4.5%.
:
between 3.0% and 4.5%.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Heavy metals, Method II  231
231 :
0.002%.
:
0.002%.
Limit of N-methylpyrrolidine—
Mobile phase—
Prepare a filtered and degassed mixture of 0.01 N nitric acid and acetonitrile (100:1). Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Column rinse solution—
Transfer 5.0 mL of nitric acid to a 1-L volumetric flask. Dilute with water to volume, and mix. Transfer this solution to an appropriate flask, add 1 L of acetonitrile, and mix.
Standard solution—
Transfer about 0.16 mL of N-methylpyrrolidine, accurately weighed, to a 100-mL volumetric flask, dissolve in and dilute with water to volume, and mix. Transfer 4.0 mL of this solution to a 100-mL volumetric flask, dilute with 0.01 N nitric acid to volume, and mix. This solution contains about 0.05 mg of N-methylpyrrolidine per mL.
Test solution—
Transfer about 100 mg of Cefepime Hydrochloride, accurately weighed, to a 10-mL volumetric flask, dissolve in and dilute with 0.01 N nitric acid to volume, and mix. [note—This solution may be kept up to 6 hours if maintained at 5 ; otherwise, use this solution within 30 minutes. ]
; otherwise, use this solution within 30 minutes. ]
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a conductivity detector and a 4.6-mm × 5-cm column that contains 5-µm packing L52. It is recommended that a 4.6-mm × 5-cm guard column containing packing L17 be placed between the pump and the injector. The flow rate is about 1 mL per minute. The typical background conductance is about 3500 µS. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the retention time of N-methylpyrrolidine is not less than 8 minutes, and the relative standard deviation for replicate injections is not more than 5.0%.
)—
The liquid chromatograph is equipped with a conductivity detector and a 4.6-mm × 5-cm column that contains 5-µm packing L52. It is recommended that a 4.6-mm × 5-cm guard column containing packing L17 be placed between the pump and the injector. The flow rate is about 1 mL per minute. The typical background conductance is about 3500 µS. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the retention time of N-methylpyrrolidine is not less than 8 minutes, and the relative standard deviation for replicate injections is not more than 5.0%.
Procedure—
Separately inject equal volumes (about 100 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the peak responses for N-methylpyrrolidine. Calculate the percentage of N-methylpyrrolidine in the portion of Cefepime Hydrochloride taken by the formula:
1000(C/W)(rU / rS)
in which C is the concentration, in mg per mL, of N-methylpyrrolidine in the Standard solution; W is the quantity, in mg, of Cefepime Hydrochloride taken to prepare the Test solution; and rU and rS are the N-methylpyrrolidine peak responses obtained from the Test solution and the Standard solution, respectively: not more than 0.3% is found. [note—Cefepime from the Test solution elutes as a broad peak at about 55 minutes. To minimize equilibration time at the start of the next day, it is recommended that the detector be turned on the night before and that Mobile phase be pumped through the system overnight at a flow rate of 0.2 mL per minute. After every Test solution injection, it is recommended that the chromatograph be flushed with Column rinse solution for 30 minutes at a flow rate of 1 mL per minute to remove cefepime from the column; and that the system then be switched back to Mobile phase at a flow rate of 1 mL per minute for reequilibration. ]
Related compounds—
Potassium phosphate solution—
Dissolve 0.68 g of monobasic potassium phosphate in 1000 mL of water.
Solution A—
Prepare a mixture of Potassium phosphate solution and acetonitrile (9:1). Adjust with potassium hydroxide or phosphoric acid to a pH of 5.0, filter, and degas.
Solution B—
Prepare a mixture of Potassium phosphate solution and acetonitrile (1:1). Adjust with potassium hydroxide or phosphoric acid to a pH of 5.0, filter, and degas.
Mobile phase—
Use variable mixtures of Solution A and Solution B as directed for Chromatographic system. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
System suitability solution—
Prepare a solution of USP Cefepime Hydrochloride System Suitability RS in Solution A containing about 1.4 mg per mL.
Test solution—
Transfer about 70 mg of Cefepime Hydrochloride, accurately weighed, to a 50-mL volumetric flask, dissolve in and dilute with Solution A to volume, sonicate, and mix. [note—Inject this solution immediately, or store in a refrigerator and inject within 12 hours. ]
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L1. The flow rate is about 1 mL per minute. The chromatograph is programmed as follows.
)—
The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L1. The flow rate is about 1 mL per minute. The chromatograph is programmed as follows.
Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the resolution, R, between cefepime and cefepime related compound A is not less than 5 and between cefepime related compound A and cefepime related compound B is not less than 10. Chromatograph the Test solution, and record the peak responses as directed for Procedure: the capacity factor, k¢, is more than 0.6; the column efficiency is not less than 4000 theoretical plates; and the tailing factor is not more than 1.5. [note—For the purpose of identification, the relative retention times are about 1.0 for cefepime, 2.7 for cefepime related compound A, and about 4.3 for cefepime related compound B. ]
| Time (minutes) |
Solution A
(%) |
Solution B
(%) |
Elution |
|---|---|---|---|
| 0–10 | 100 | 0 | isocratic |
| 10–30 | 100®50 | 0®50 | linear gradient |
| 30–35 | 50 | 50 | isocratic |
| 35–36 | 50®100 | 50®0 | linear gradient |
Procedure—
Inject a volume (about 10 µL) of the Test solution into the chromatograph, record the chromatogram, and measure the peak responses. Calculate the percentage of each impurity in the portion of Cefepime Hydrochloride taken by the formula:
100(ri / rs)
in which ri is the peak response for each impurity; and rs is the sum of the responses for all the peaks: not more than 0.3% of cefepime related compound A is found; not more than 0.2% of cefepime related compound B is found; and not more than 0.1% of any other impurity is found.
Other requirements—
Where the label states that Cefepime Hydrochloride is sterile, it meets the requirements for Sterility under Cefepime for Injection.
Assay—
Mobile phase—
Dissolve 5.76 g of sodium 1-pentanesulfonate in 2000 mL of water. Adjust with glacial acetic acid to a pH of 3.4, and then with potassium hydroxide TS to a pH of 4.0. Prepare a filtered and degassed mixture of this solution and acetonitrile (94:6). Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard preparation—
Dissolve an accurately weighed quantity of USP Cefepime Hydrochloride RS in Mobile phase to obtain a solution having a known concentration of about 1.4 mg per mL.
Assay preparation—
Transfer about 70 mg of Cefepime Hydrochloride, accurately weighed, to a 50-mL volumetric flask, dissolve in and dilute with Mobile phase to volume, and mix.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 254-nm detector and a 3.9-mm × 30-cm column that contains packing L1. The flow rate is about 2 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the column efficiency is not less than 1500 theoretical plates; the tailing factor is not more than 1.7; and the relative standard deviation for replicate injections is not more than 2.0%.
)—
The liquid chromatograph is equipped with a 254-nm detector and a 3.9-mm × 30-cm column that contains packing L1. The flow rate is about 2 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the column efficiency is not less than 1500 theoretical plates; the tailing factor is not more than 1.7; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 10 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in µg, of cefepime (C19H24N6O5S2) in each mg of Cefepime Hydrochloride taken by the formula:
50(CP/W)(rU/rS)
in which C is the concentration, in mg per mL, of USP Cefepime Hydrochloride RS in the Standard preparation; P is the content, in µg per mg, of cefepime in USP Cefepime Hydrochloride RS; W is the weight, in mg, of Cefepime Hydrochloride taken to prepare the Assay preparation; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ahalya Wise, M.S.
Senior Scientific Liaison 1-301-816-8161 |
(SM12010) Monographs - Small Molecules 1 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
|
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology |
USP35–NF30 Page 2544
Pharmacopeial Forum: Volume No. 36(1) Page 76