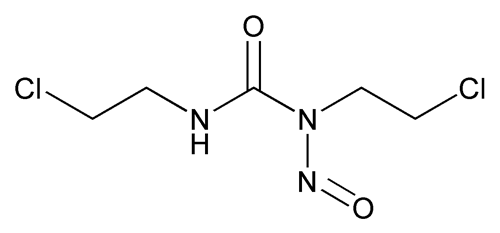
Carmustine
C5H9Cl2N3O2 214.05
Urea, N,N'-bis(2-chloroethyl)-N-nitroso-;
1,3-Bis(2-chloroethyl)-1-nitrosourea

 [154-93-8].
[154-93-8].
(kar mus' teen).
C5H9Cl2N3O2 214.05
Urea, N,N'-bis(2-chloroethyl)-N-nitroso-;
1,3-Bis(2-chloroethyl)-1-nitrosourea
DEFINITION
Carmustine contains NLT 98.0% and NMT 102.0% of C5H9Cl2N3O2, calculated on the anhydrous and solvent-free basis.
[Caution—Use appropriate surgical gloves, arm covers, and a dust mask. Perform all work under a fume hood approved for testing cytotoxic agents when possible.
]
IDENTIFICATION
• A. Infrared Absorption  197F
197F
Sample:
Melt a small portion of the sample in a suitable container in a controlled water bath or oven, and set the temperature between 33 and 40
and 40 .
.
Standard:
A similar preparation of USP Carmustine RS
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
[Note—Prepare solutions in low-actinic glassware, and keep them refrigerated until use. ]
Mobile phase:
Acetonitrile and water (3:7)
Standard solution:
1.5 mg/mL of USP Carmustine RS in acetonitrile
Sample solution:
1.5 mg/mL of Carmustine in acetonitrile
Chromatographic system
Mode:
LC
Detector:
UV 200 nm
Refrigerated autosampler temperature:
4 –5
–5
Column:
4.6-mm × 15-cm; 5-µm packing L1
Flow rate:
1.5 mL/min
Injection size:
10 µL
System suitability
Sample:
Standard solution
Suitability requirements
Tailing factor:
NMT 1.9
Relative standard deviation:
NMT 2.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C5H9Cl2N3O2 in the portion of Carmustine taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Carmustine RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Carmustine in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–102.0% on the anhydrous and solvent-free basis
IMPURITIES
Inorganic Impurities
• Heavy Metals, Method II  231
231 :
NMT 20 ppm
:
NMT 20 ppm
Organic Impurities
• Procedure 1: Limit of Ether-Insoluble Substances
[Note—Perform in a well-ventilated fume hood. ]
Analysis:
Transfer 1.0 g of sample to a suitable container containing 10 mL of anhydrous ether, stir for 5 min, and immediately filter through a tared glass-filtering crucible of medium pore size. Wash the container with an additional 10 mL of ether, and filter through the same glass-filtering crucible. Dry the crucible at 105 for 1 h. Cool in a desiccator and weigh.
for 1 h. Cool in a desiccator and weigh.
Acceptance criteria:
The weight of the residue does not exceed 0.1%.
• Procedure 2: Limit of Carmustine Related Compound A
[Note—Prepare solutions in low-actinic glassware, and keep them refrigerated until use. ]
Mobile phase, Sample solution, and Chromatographic system:
Proceed as directed in the Assay.
Carmustine standard solution:
Use the Standard solution, prepared as directed in the Assay.
Standard stock solution:
0.75 mg/mL of USP Carmustine Related Compound A RS in acetonitrile
Standard solution:
0.0075 mg/mL of USP Carmustine Related Compound A RS in acetonitrile, from the Standard stock solution
System suitability solution 1:
0.75 µg/mL of USP Carmustine Related Compound A RS in acetonitrile, from the Standard solution
System suitability solution 2:
Transfer 5.0 mL of Carmustine standard solution and 10.0 mL of Standard stock solution into a 100-mL volumetric flask, and dilute with acetonitrile to volume. Transfer 5.0 mL of this solution into a 50-mL volumetric flask, and dilute with acetonitrile to volume.
System suitability
Samples:
Carmustine standard solution, System suitability solution 1, and System suitability solution 2
[Note—The relative retention times for carmustine related compound A and carmustine are 0.3 and 1.0, respectively. ]
Suitability requirements
Resolution:
NLT 10 between carmustine related compound A and carmustine, System suitability solution 2
Tailing factor:
NMT 1.9, Carmustine standard solution
Relative standard deviation:
NMT 5%, System suitability solution 1
Analysis
[Note—Run the Sample solution at least 1.5 times the retention time of carmustine. ]
Samples:
Standard solution and Sample solution
Calculate the percentage of carmustine related compound A in the portion of Carmustine taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of carmustine related compound A from the Sample solution |
| rS | = | = peak response of carmustine related compound A from the Standard solution |
| CS | = | = concentration of carmustine related compound A in the Standard solution (mg/mL) |
| CU | = | = concentration of Carmustine in the Sample solution (mg/mL) |
Calculate the percentage of each unspecified impurity in the portion of Carmustine taken:
Result = (rU/rT) × 100
| rU | = | = peak response of any unspecified impurity from the Sample solution |
| rT | = | = sum of all peak responses from the Sample solution |
Acceptance criteria
Carmustine related compound A:
NMT 0.5%
Any unspecified impurity:
NMT 0.1%
• Procedure 3: Limit of 2-Chloroethylamine
[Note—Prepare solutions in low-actinic glassware, and keep them refrigerated until use. ]
Standard solution 1 (0.2%):
1.2 mg/mL of 2-chloroethylamine monohydrochloride in methanol. [Note—1.2 mg/mL of 2-chloroethylamine monohydrochloride is equivalent to 0.8 mg/mL of 2-chloroethylamine. ]
Standard solution 2 (0.1%):
0.4 mg/mL of USP Carmustine RS in methanol
Sample solution:
0.4 g/mL of Carmustine in methanol
Chromatographic system
Mode:
TLC
Adsorbent:
0.25-mm layer of chromatographic plate (20-cm × 20-cm) coated with silica gel 60
Application volume:
1 µL
Developing solvent system 1:
Ethyl acetate
Developing solvent system 2:
Ethyl acetate and methanol (7:3)
Spray reagent 1:
Diethylamine
Spray reagent 2:
0.1 N silver nitrate solution
Analysis
Samples:
Standard solution 1 (0.2%), Standard solution 2 (0.1%), and Sample solution
Develop with Developing solvent system 1 for 27 min, followed by air drying for 5 min. Develop again in Developing solvent system 2 for 8 min, followed by air drying for 10 min. Spray the plate with Spray reagent 1, and heat the plate for 20 min in an oven at 100 . Allow the plates to cool to room temperature, and spray the plate with Spray reagent 2. Allow the plate to be exposed to UV light at 365 nm for 15 min. Examine the plate under UV light.
. Allow the plates to cool to room temperature, and spray the plate with Spray reagent 2. Allow the plate to be exposed to UV light at 365 nm for 15 min. Examine the plate under UV light.
Acceptance criteria
2-Chloroethylamine:
The spot for 2-chloroethylamine from the Sample solution is not more intense than the principal spot from Standard solution 1 (0.2%).
Any unspecified impurity:
Any spot if present in the chromatogram from the Sample solution, except the principal spot of carmustine and the spot of 2-chloroethylamine, is not more intense than the principal spot from Standard solution 2 (0.1%).
• Procedure 4: Limit of 2-Chloroethanol
Standard solution:
0.02 mg/mL of 2-chloroethanol in acetonitrile
System suitability solution:
0.01 mg/mL of 2-chloroethanol in acetonitrile, diluted from the Standard solution
Sample solution:
10 mg/mL of Carmustine in acetonitrile. [Note—Prepare in low-actinic glassware, and keep refrigerated until use. ]
Chromatographic system
Mode:
GC
Detector:
Flame ionization
Column:
30-m × 0.53-mm column bonded with a 1-µm film of phase G16
Temperature
Injector:
90
Detector:
260
Column:
See the temperature program table below.
| Initial Temperature ( |
Temperature Ramp ( |
Final Temperature ( |
Hold Time at Final Temperature (min) |
|---|---|---|---|
| 40 | 0 | 40 | 6 |
| 40 | 30 | 80 | 14 |
| 80 | 30 | 200 | 3 |
Carrier gas:
Helium
Flow rate:
7 mL/min
Injection size:
5 µL
System suitability
Sample:
System suitability solution
Suitability requirements
Relative standard deviation:
NMT 5%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of 2-chloroethanol in the portion of Carmustine taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of 2-chloroethanol from the Sample solution |
| rS | = | = peak response of 2-chloroethanol from the Standard solution |
| CS | = | = concentration of 2-chloroethanol in the Standard solution (mg/mL) |
| CU | = | = concentration of Carmustine in the Sample solution (mg/mL) |
Acceptance criteria
2-Chloroethanol:
NMT 0.1%
• Procedure 5: Limit of Acetaldehyde
Standard solution:
10 µg/mL of acetaldehyde in acetonitrile
Sample solution:
10 mg/mL of Carmustine in acetonitrile. [Note—Prepare in low-actinic glassware, and keep refrigerated until use. ]
Chromatographic system
Mode:
GC
Detector:
Flame ionization
Column:
30-m × 0.53-mm column bonded with a 5-µm film of phase G1
Temperature
Injector:
70
Detector:
260
Column:
See the temperature program table below.
| Initial Temperature ( |
Temperature Ramp ( |
Final Temperature ( |
Hold Time at Final Temperature (min) |
|---|---|---|---|
| 40 | 0 | 40 | 6 |
| 40 | 30 | 210 | 3 |
Injector split ratio:
15:1
Carrier gas:
Helium
Flow rate:
3 mL/min
Injection size:
5 µL
System suitability
Sample:
Standard solution
Suitability requirements
Relative standard deviation:
NMT 5%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of acetaldehyde in the portion of Carmustine taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of acetaldehyde from the Sample solution |
| rS | = | = peak response of acetaldehyde from the Standard solution |
| CS | = | = concentration of acetaldehyde in the Standard solution (mg/mL) |
| CU | = | = concentration of Carmustine in the Sample solution (mg/mL) |
Acceptance criteria
Acetaldehyde:
NMT 0.1%
SPECIFIC TESTS
• Water Determination, Method I  921
921 :
NMT 0.5%
:
NMT 0.5%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight, light-resistant containers at a temperature between 2 and 8
and 8 .
.
• USP Reference Standards  11
11
USP Carmustine Related Compound A RS
1,3-Bis(2-chloroethyl) urea.
C5H10Cl2N2O 185.05
1,3-Bis(2-chloroethyl) urea.
C5H10Cl2N2O 185.05
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Feiwen Mao, M.S.
Senior Scientific Liaison 1-301-816-8320 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2504
Pharmacopeial Forum: Volume No. 35(3) Page 546