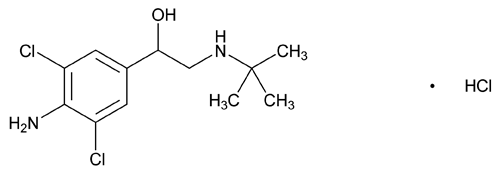
Clenbuterol Hydrochloride
C12H18Cl2N2O·HCl 313.65
Ethanol, 1-(4-amino-3,5-dichlorophenyl)-2-(tert-butylamino), hydrochloride;
4-Amino- -[(tert-butylamino)methyl]-3,5-dichlorobenzyl alcohol, hydrochloride
-[(tert-butylamino)methyl]-3,5-dichlorobenzyl alcohol, hydrochloride


 [21898-19-1].
[21898-19-1].
C12H18Cl2N2O·HCl 313.65
Ethanol, 1-(4-amino-3,5-dichlorophenyl)-2-(tert-butylamino), hydrochloride;
4-Amino-
DEFINITION
Clenbuterol Hydrochloride contains NLT 98.0% and NMT 102.0% of C12H18Cl2N2O·HCl, calculated on the anhydrous basis.
IDENTIFICATION
• A. Infrared Absorption  197K
197K
[Note—Alternatively, Infrared Absorption  197A
197A may be used. ]
may be used. ]
• B. Identification Tests—General, Chloride  191
191 :
Meets the requirements
:
Meets the requirements
ASSAY
• Procedure
Sample solution:
Dissolve 0.25 g in 50 mL of alcohol, and add 5.0 mL of 0.01 N hydrochloric acid.
Analysis:
Titrate with 0.1 N sodium hydroxide VS, determining the endpoint potentiometrically. Read the volume added between the two points of inflection. Perform a blank determination, and make any necessary correction (see Titrimetry  541
541 ). Each mL of 0.1 N sodium hydroxide is equivalent to 31.37 mg of C12H18Cl2N2O·HCl.
). Each mL of 0.1 N sodium hydroxide is equivalent to 31.37 mg of C12H18Cl2N2O·HCl.
Acceptance criteria:
98.0%–102.0% on the anhydrous basis
IMPURITIES
Inorganic Impurities
• Residue on Ignition  281
281 :
NMT 0.1%, from 1–2 g
:
NMT 0.1%, from 1–2 g
• Heavy Metals, Method II  231
231 :
NMT 10 ppm
:
NMT 10 ppm
Organic Impurities
• Procedure
Buffer:
Dissolve 3.0 g of sodium 1-decanesulfonate and 5.0 g of monobasic potassium phosphate in 900 mL of water, adjust with dilute phosphoric acid (1 in 10) to a pH of 3.0, and dilute with water to 1000 mL.
Mobile phase:
Acetonitrile, methanol, and Buffer (2:2:6)
System suitability solution:
0.2 mg/mL each of USP Clenbuterol Related Compound B RS and Clenbuterol Hydrochloride in Mobile phase
Sample solution 1:
2.0 mg/mL of Clenbuterol Hydrochloride in Mobile phase
Sample solution 2:
2.0 µg/mL of Clenbuterol Hydrochloride in Mobile phase, from Sample solution 1
Chromatographic system
Mode:
LC
Detector:
UV 215 nm
Column:
4.0-mm × 12.5-cm; 5-µm packing L1
Column temperature:
40
Flow rate:
0.5 mL/min
Injection size:
5 µL
System suitability
Sample:
System suitability solution
Suitability requirements
Resolution:
NLT 4.0 between clenbuterol related compound B and clenbuterol
Relative standard deviation:
NMT 2.0% for the clenbuterol peak
Analysis
Samples:
Sample solution 1 and Sample solution 2
Calculate the percentage of impurities in the portion of C12H18Cl2N2O·HCl taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of each impurity from Sample solution 1 |
| rS | = | = peak response of clenbuterol from Sample solution 2 |
| CS | = | = concentration of Clenbuterol Hydrochloride in Sample solution 2 (mg/mL) |
| CU | = | = concentration of Clenbuterol Hydrochloride in Sample solution 1 (mg/mL) |
Acceptance criteria
Individual impurities:
0.1%
Total impurities:
NMT 0.2%
[Note—The reporting level for impurities is 0.05%. ]
SPECIFIC TESTS
• Optical Rotation, Specific Rotation  781S
781S
Sample:
30 mg/mL in water, filter as necessary
Acceptance criteria:
 10
10 to +10
to +10 at 20
at 20
• Water Determination, Method I  921
921 :
NMT 1.0%
:
NMT 1.0%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed containers, protected from light. Store at room temperature.
• Labeling:
Label it to indicate that it is for veterinary use only.
• USP Reference Standards  11
11
USP Clenbuterol Related Compound B RS
1-(4-Amino-3,5-dichlorophenyl)-2-tert-butyl-aminoethanone hydrochloride.
C12H16Cl2N2O·HCl 311.64
1-(4-Amino-3,5-dichlorophenyl)-2-tert-butyl-aminoethanone hydrochloride.
C12H16Cl2N2O·HCl 311.64
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Morgan Puderbaugh, B.S.
Associate Scientific Liaison 1-301-998-6833 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2700
Pharmacopeial Forum: Volume No. 35(5) Page 1125