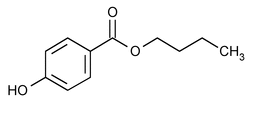
Butylparaben
(bue'' til par' a ben).
» Butylparaben contains not less than 98.0 percent and not more than 102.0 percent of C11H14O3.
Packaging and storage—
Preserve in well-closed containers.
Color of solution—
Dissolve 1 g in alcohol, dilute with alcohol to 10 mL, and mix (Butylparaben solution). This solution is clear and not more intensely colored than alcohol or a solution prepared immediately before use by mixing 2.4 mL of ferric chloride CS, 1.0 mL of cobaltous chloride CS, and 0.4 mL of cupric sulfate CS with 0.3 N hydrochloric acid to make 10 mL, and diluting 5 mL of this solution with 0.3 N hydrochloric acid to make 100 mL. Make the comparison by viewing the solutions downward in matched color-comparison tubes against a white surface (see Color and Achromicity  631
631 ).
).
Acidity—
To 2 mL of Butylparaben solution prepared in the Color of solution test, add 3 mL of alcohol, 5 mL of carbon dioxide-free water, and 0.1 mL of bromocresol green TS, and titrate with 0.10 N sodium hydroxide: not more than 0.1 mL is required to produce a blue color.
Residue on ignition  281
281 :
not more than 0.1%, determined on 1.0 g.
:
not more than 0.1%, determined on 1.0 g.
Related substances—
Test solution—
Prepare a solution of Butylparaben in acetone containing 10 mg per mL.
Standard solutions—
Transfer 0.5 mL of the Test solution to a 100-mL volumetric flask, dilute with acetone to volume, and mix (Standard solution A). Dissolve 10 mg, accurately weighed, of USP Propylparaben RS in 1 mL of the Test solution, and dilute with acetone to 10 mL (Standard solution B).
Procedure—
Separately apply 2 µL of the Test solution and 2 µL of each Standard solution to a thin-layer chromatographic plate (see Chromatography  621
621 ), coated with a 0.25-mm layer of chromatographic octadecylsilanized silica gel mixture. Develop the chromatogram in a solvent system consisting of a mixture of methanol, water, and glacial acetic acid (70:30:1) until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the chamber, mark the solvent front, and allow the solvent to evaporate. Examine the plate under short-wavelength UV light, and compare the intensities of any secondary spots observed in the chromatogram of the Test solution with that of the principal spot in the chromatogram of Standard solution A: the intensity of any individual secondary spot in the chromatogram of the Test solution is not greater than that of the principal spot obtained in the chromatogram of Standard solution A (0.5%). The test is not valid unless the chromatogram obtained with Standard solution B shows two clearly separated principal spots.
), coated with a 0.25-mm layer of chromatographic octadecylsilanized silica gel mixture. Develop the chromatogram in a solvent system consisting of a mixture of methanol, water, and glacial acetic acid (70:30:1) until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the chamber, mark the solvent front, and allow the solvent to evaporate. Examine the plate under short-wavelength UV light, and compare the intensities of any secondary spots observed in the chromatogram of the Test solution with that of the principal spot in the chromatogram of Standard solution A: the intensity of any individual secondary spot in the chromatogram of the Test solution is not greater than that of the principal spot obtained in the chromatogram of Standard solution A (0.5%). The test is not valid unless the chromatogram obtained with Standard solution B shows two clearly separated principal spots.
Assay—
To about 1.000 g of Butylparaben, accurately weighed, add 20.0 mL of 1 N sodium hydroxide VS, and heat at about 70 for 1 hour. Cool rapidly in an ice bath. Carry out the titration on the solutions at room temperature. Titrate the excess sodium hydroxide with 1 N sulfuric acid VS, continuing the titration until the second point of inflection and determining the endpoint potentiometrically (see Titrimetry
for 1 hour. Cool rapidly in an ice bath. Carry out the titration on the solutions at room temperature. Titrate the excess sodium hydroxide with 1 N sulfuric acid VS, continuing the titration until the second point of inflection and determining the endpoint potentiometrically (see Titrimetry  541
541 ). Perform a blank determination (see Residual Titrations under Titrimetry
). Perform a blank determination (see Residual Titrations under Titrimetry  541
541 ). Each mL of 1 N sodium hydroxide is equivalent to 194.2 mg of C11H14O3.
). Each mL of 1 N sodium hydroxide is equivalent to 194.2 mg of C11H14O3.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Robert H. Lafaver, M.S.
Scientific Liaison 1-301-816-8335 |
(EXC2010) Monographs - Excipients |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 1718
Pharmacopeial Forum: Volume No. 34(6) Page 1592