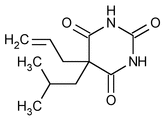
Butalbital
(bue tal' bi tal).
2,4,6(1H,3H,5H)-Pyrimidinetrione, 5-(2-methylpropyl)-5-(2-propenyl)-.
5-Allyl-5-isobutylbarbituric acid
» Butalbital contains not less than 98.0 percent and not more than 102.0 percent of C11H16N2O3, calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers.
Identification—
B:
Ultraviolet Absorption  197U
197U —
—
Solution:
15 µg per mL.
Medium:
0.1 N sodium hydroxide.
Absorptivities at 246 nm, calculated on the dried basis, do not differ by more than 2.5%.
Melting range  741
741 :
between 138
:
between 138 and 141
and 141 .
.
Loss on drying  731
731 —
Dry it in vacuum at room temperature to constant weight: it loses not more than 0.2% of its weight.
—
Dry it in vacuum at room temperature to constant weight: it loses not more than 0.2% of its weight.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Heavy metals, Method II  231
231 :
0.002%.
:
0.002%.
Chromatographic purity—
Chloroform-methanol—
Mix equal volumes of chloroform and methanol.
Standard preparations—
Dissolve a quantity of USP Butalbital RS in Chloroform-methanol to obtain a solution having a concentration of 40 mg per mL (Solution A). Dilute 1.0 mL of Solution A with Chloroform-methanol to 100 mL, and mix (Solution B); mix 5.0 mL of Solution B with 5.0 mL of Chloroform-methanol (Solution C); and mix 5.0 mL of Solution C with 5.0 mL of Chloroform-methanol (Solution D).
Test preparation—
Dissolve a quantity of Butalbital in Chloroform-methanol to obtain a solution having a concentration of 40 mg per mL.
Procedure—
In a suitable chromatographic chamber, arranged for thin-layer chromatography and lined with filter paper, place a volume of a developing solvent consisting of a mixture of acetone, dichloromethane, methanol, and ammonium hydroxide (50:30:10:10) sufficient to develop the chromatogram. Cover the chamber, and allow it to equilibrate for 30 minutes. Apply 10 µL each of the Test preparation and Solutions A, B, C, and D to a suitable thin-layer chromatographic plate (see Chromatography  621
621 ) coated with a 0.25-mm layer of chromatographic silica gel mixture. Develop the chromatogram until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the developing chamber, mark the solvent front, and dry the plate in a current of air. Spray the plate with a reagent prepared by dissolving 5 g of potassium hydroxide in a mixture of 25 mL of water and 75 mL of alcohol. Allow the plate to dry in warm air for 10 minutes, and examine the chromatograms under UV light: the chromatograms show principal spots at about the same RF value; and the sum of the intensities of any secondary spots, if present in the chromatogram from the Test preparation, is not greater than 1% of that of the principal spot from Solution A. [note—The relative intensities of the principal spots from the Standard preparations are: Solution A, 1; Solution B, 0.01; Solution C, 0.005; and Solution D, 0.0025. ]
) coated with a 0.25-mm layer of chromatographic silica gel mixture. Develop the chromatogram until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the developing chamber, mark the solvent front, and dry the plate in a current of air. Spray the plate with a reagent prepared by dissolving 5 g of potassium hydroxide in a mixture of 25 mL of water and 75 mL of alcohol. Allow the plate to dry in warm air for 10 minutes, and examine the chromatograms under UV light: the chromatograms show principal spots at about the same RF value; and the sum of the intensities of any secondary spots, if present in the chromatogram from the Test preparation, is not greater than 1% of that of the principal spot from Solution A. [note—The relative intensities of the principal spots from the Standard preparations are: Solution A, 1; Solution B, 0.01; Solution C, 0.005; and Solution D, 0.0025. ]
Assay—
Dissolve about 180 mg of Butalbital, accurately weighed, in a mixture of 25 mL of alcohol and 25 mL of sodium carbonate solution (3 in 100), and titrate with 0.1 N silver nitrate VS, determining the endpoint electrometrically, using a silver electrode, either with a suitable reference electrode containing a saturated aqueous solution of potassium nitrate, or a combination electrode in which the reference portion of the electrode contains a saturated aqueous solution of potassium nitrate. Each mL of 0.1 N silver nitrate is equivalent to 22.43 mg of C11H16N2O3.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ravi Ravichandran, Ph.D.
Principal Scientific Liaison 1-301-816-8330 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2413