The following methods are provided for the determination of sulfur dioxide in pharmaceutical excipients.
METHOD I
Procedure
Mix 20 g of the test specimen, accurately weighed, with 200 mL of an appropriate solvent as indicated in each individual monograph, and stir until a smooth suspension is obtained. Allow the test specimen mixture to remain undisturbed until most of the test specimen has settled, and filter the aqueous portion through paper (Whatman No. 1 or equivalent). To 100 mL of the clear filtrate add an additional solvent as indicated in each individual monograph, add 3 mL of starch TS, and titrate with 0.01 N iodine solution VS to the first permanent blue or purple color. Each 1.0 mL of 0.01 N iodine solution VS consumed corresponds to 0.003% of sulfur dioxide found.
METHOD II
Procedure
Transfer about 50 to 100 g of the substance to be tested, accurately weighed, to a 250-mL conical flask, add 100 to 150 mL of water, and mix. Cool to between 5 and 10
and 10 . While stirring with a magnetic stirrer, add 10 mL of cold 1.5 N sodium hydroxide (at a temperature between 5
. While stirring with a magnetic stirrer, add 10 mL of cold 1.5 N sodium hydroxide (at a temperature between 5 and 10
and 10 ). Stir for an additional 20 seconds, and add 10 mL of starch indicator solution, prepared as follows: mix 10 g of soluble starch with 50 mL of cold water, transfer to 1000 mL of boiling water, stir until completely dissolved, cool, and add 1 g of salicylic acid preservative. [Note—Discard the solution after 1 month. ] Add 10 mL of 2.0 N sulfuric acid (at a temperature between 5
). Stir for an additional 20 seconds, and add 10 mL of starch indicator solution, prepared as follows: mix 10 g of soluble starch with 50 mL of cold water, transfer to 1000 mL of boiling water, stir until completely dissolved, cool, and add 1 g of salicylic acid preservative. [Note—Discard the solution after 1 month. ] Add 10 mL of 2.0 N sulfuric acid (at a temperature between 5 and 10
and 10 ), and titrate immediately with 0.005 N iodine VS until a light blue color persists for 1 minute (see Titrimetry
), and titrate immediately with 0.005 N iodine VS until a light blue color persists for 1 minute (see Titrimetry  541
541 ). Perform a blank determination, using 200 mL of water treated similarly to the solution under test, and make any necessary correction. Each mL of 0.005 N iodine is equivalent to 0.16 mg of SO2.
). Perform a blank determination, using 200 mL of water treated similarly to the solution under test, and make any necessary correction. Each mL of 0.005 N iodine is equivalent to 0.16 mg of SO2.
METHOD III
Procedure
Dissolve 20.0 g of the test specimen in 150 mL of hot water in a flask having a round bottom and a long neck, add 5 mL of phosphoric acid and 1 g of sodium bicarbonate, and at once connect the flask to a condenser. [Note—Excessive foaming can be alleviated by the addition of a few drops of a suitable antifoaming agent. ] Distill 50 mL, receiving the distillate under the surface of 50 mL of 0.1 N iodine. Acidify the distillate with a few drops of hydrochloric acid, add 2 mL of barium chloride TS, and heat on a steam bath until the liquid is nearly colorless. The precipitate of barium sulfate, if any, when filtered, washed, and ignited, weighs not more than 3 mg, corresponding to not more than 0.004% of sulfur dioxide, correction being made for any sulfate that may be present in 50 mL of the 0.1 N iodine.
METHOD IV
In this test, sulfur dioxide is released from the test specimen in a boiling acid medium and is removed by a stream of carbon dioxide. The separated gas is collected in a dilute hydrogen peroxide solution, in which the sulfur dioxide is oxidized to sulfuric acid and titrated with standard alkali, using a pH meter to control the pH value and titration. This test is performed under conditions such that the requirements specified in the system suitability test are met.
Special Reagents
Carbon Dioxide—
Use carbon dioxide with a flow regulator that will maintain a flow of 100 ± 10 mL per minute.
Hydrogen Peroxide Solution—
Dilute 30% hydrogen peroxide with water to obtain a 3% solution. Neutralize the 3% hydrogen peroxide solution with 0.01 N sodium hydroxide to a pH of 4.1 determined potentiometrically.
Potassium Metabisulfite Solution—
Transfer 0.87 g of potassium metabisulfite (K2S2O5) and 0.2 g of edetate disodium to a 1000-mL volumetric flask. Dilute with water to volume before mixing. [Note—Edetate disodium is used to protect sulfite ion from oxidation. ]
Apparatus
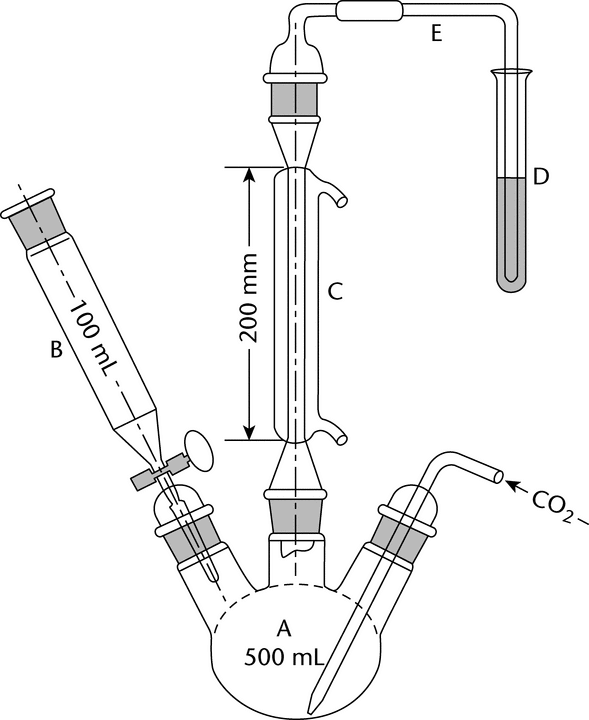
A suitable apparatus for sulfur dioxide determination is shown in the accompanying diagram (Figure 1).
The apparatus consists of a 500-mL three-neck, round-bottom boiling flask, A; a separatory funnel, B, having a capacity of 100 mL or greater; a gas inlet tube of sufficient length to permit introduction of the carbon dioxide within 2.5 cm of the bottom of the boiling flask; a reflux condenser, C, having a jacket length of 200 mm; and a delivery tube, E, connecting the upper end of the reflux condenser to the bottom of a receiving test tube, D. Apply a thin film of stopcock grease to the sealing surfaces of all joints except the joint between the separatory funnel and the boiling flask, and clamp the joints to ensure tightness.
System Suitability Test
Test A—
Using the Potassium Metabisulfite Solution as the standard, proceed as directed for Procedure, except replace the 25.0 g of test substance with 20 mL of Potassium Metabisulfite Solution. Calculate the content, in µg per mL, of sulfur dioxide in the Potassium Metabisulfite Solution taken by the formula:
1000(32.03)VN/VP
in which the factor 1000 converts mg to µg; 32.03 is the milliequivalent weight of sulfur dioxide; V is the volume, in mL, of titrant consumed; N is the normality of the titrant; and VP is the volume, in mL, of the Potassium Metabisulfite Solution taken for the test.
Test B—
In a 100-mL conical flask, add 20 mL of 0.02 N iodine solution and 5 mL of 2 N hydrochloric acid. Add 1 mL of starch TS, and titrate with the Potassium Metabisulfite Solution until the first discoloration is observed. Calculate the content, in µg per mL, of sulfur dioxide in the Potassium Metabisulfite Solution by the formula:
1000(32.03)VINI/VP
in which 1000 and 32.03 are defined above; VI is the volume, in mL, of the iodine solution used in the test; NI is the normality of the iodine solution; and VP is the volume, in mL, of the Potassium Metabisulfite Solution consumed.
The difference between the sulfur dioxide contents obtained from Test A and Test B is not more than 5% of their mean value. Test B shall be performed within 15 minutes after completion of Test A. [Note—This avoids a potential variation of the sulfur dioxide content in the Potassium Metabisulfite Solution when stored at room temperature. ]
Procedure
Add 150 mL of water to the boiling flask (A). Close the stopcock of the separatory funnel, and begin the flow of carbon dioxide at a rate of 100 ± 5 mL per minute through the apparatus. Start the condenser coolant flow. Place 10 mL of Hydrogen Peroxide Solution in the receiving test tube (D). After 15 minutes, without interrupting the flow of carbon dioxide, remove the separatory funnel (B) from the boiling flask, and transfer 25.0 g of the test specimen to the boiling flask with the aid of 100 mL of water. Apply stopcock grease to the outer joint of the separatory funnel, and replace the separatory funnel in the boiling flask. Close the stopcock of the separatory funnel, and add 80 mL of 2 N hydrochloric acid to the separatory funnel. Open the stopcock of the separatory funnel to permit the hydrochloric acid solution to flow into the boiling flask, guarding against escape of sulfur dioxide into the separatory funnel by closing the stopcock before the last few mL of hydrochloric acid drain out. Boil the mixture for 1 hour. Open the stopcock of the funnel, stop the flow of carbon dioxide, discontinue heating the flask, and turn off the cooling water in the condenser. Remove the receiving test tube, and transfer its contents to a 200-mL wide-necked, conical flask. Rinse the receiving test tube with a small portion of water, add the rinsing to the 200-mL conical flask, and mix. Heat on a water bath for 15 minutes, and allow to cool. Add 0.1 mL of bromophenol blue TS, and titrate the contents with 0.1 N sodium hydroxide VS until the color changes from yellow to violet-blue, with the color change lasting for at least 20 seconds. Perform a blank determination and make any necessary correction (see Titrimetry  541
541 ). Calculate the content, in µg per g, of sulfur dioxide in the test specimen taken by the formula:
). Calculate the content, in µg per g, of sulfur dioxide in the test specimen taken by the formula:
1000(32.03)VN/W
in which the factor 1000 converts mg to µg; 32.03 is the milliequivalent weight of sulfur dioxide; V is the volume, in mL, of titrant consumed; N is the normality of the titrant; and W is the weight, in g, of the test specimen taken.
METHOD V
In this method, similar to Method IV, sulfur dioxide is released from the test specimen in a boiling acid medium and is removed by a stream of nitrogen. The separated gas is collected in a dilute hydrogen peroxide solution, in which the sulfur dioxide is oxidized to sulfuric acid and titrated with standard alkali, using methyl red as an indicator. This test is performed under conditions such that the requirements specified in the system suitability test are met.
Special Reagents
Hydrogen Peroxide Solution—
Dilute a portion of 30 percent hydrogen peroxide with water to obtain a 3% solution. Just before use, add 3 drops of methyl red TS, and neutralize to a yellow endpoint with 0.01 N sodium hydroxide. Do not exceed the endpoint.
Nitrogen—
Use high-purity nitrogen with a flow regulator that will maintain a flow of 200 ± 10 mL per minute. Guard against the presence of oxygen by passing the nitrogen through a scrubber, such as alkaline pyrogallol, prepared as follows: add 4.5 g of pyrogallol to a gas-washing bottle, purge the bottle with nitrogen for 3 minutes, and add a solution containing 85 mL of water and 65 g of potassium hydroxide while maintaining an atmosphere of nitrogen in the bottle.
Potassium Metabisulfite Solution—
Transfer 0.87 g of potassium metabisulfite (K2S2O5) and 0.2 g of edetate disodium to a 1000-mL volumetric flask. Dilute with water to volume before mixing. [Note—Edetate disodium is used to protect sulfite ion from oxidation. ]
Apparatus
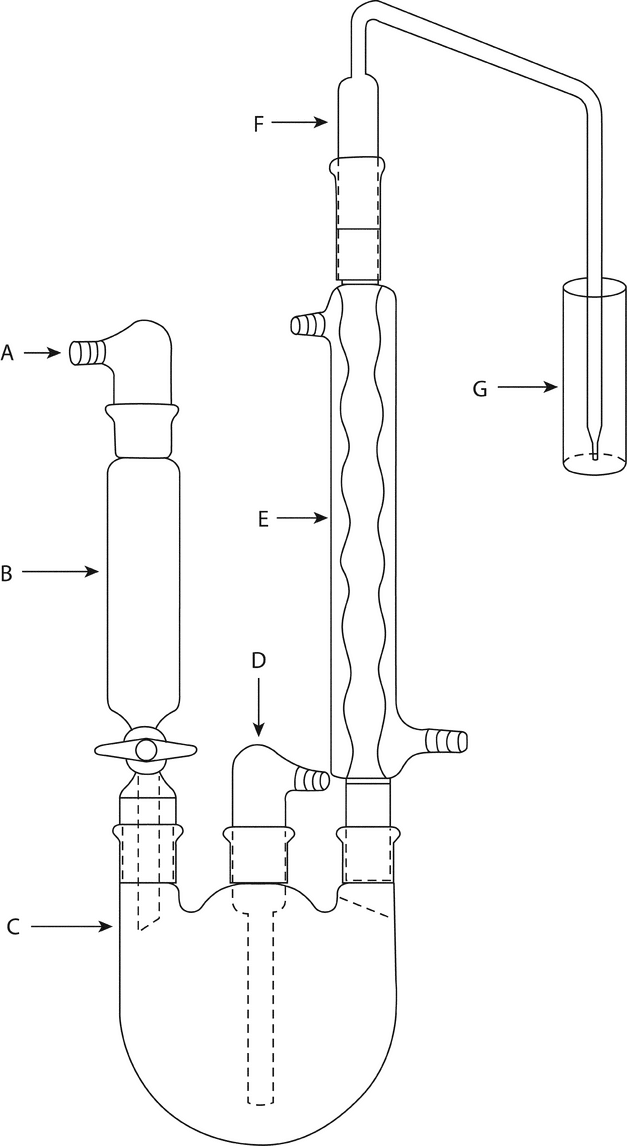
The apparatus (see Figure 2) is designed to effect the selective transfer of sulfur dioxide from the specimen in boiling aqueous hydrochloric acid to the Hydrogen Peroxide Solution in vessel G. The backpressure is limited to the unavoidable pressure due to the height of the Hydrogen Peroxide Solution above the tip of the bubbler, F. Keeping the backpressure as low as possible reduces the likelihood that sulfur dioxide will be lost through leaks. Preboil vinyl and silicone tubing. Apply a thin film of stopcock grease to the sealing surfaces of all joints, except the joint between the separatory funnel and the flask, and clamp the joints to ensure tightness. The separatory funnel, B, has a capacity of 100 mL or greater. The inlet adapter, A, with a hose connector, provides a means of applying headpressure over the solution. [Note—A pressure-equalizing dropping funnel is not recommended because condensate, which may contain sulfur dioxide, is deposited in the funnel and the side arm. ]
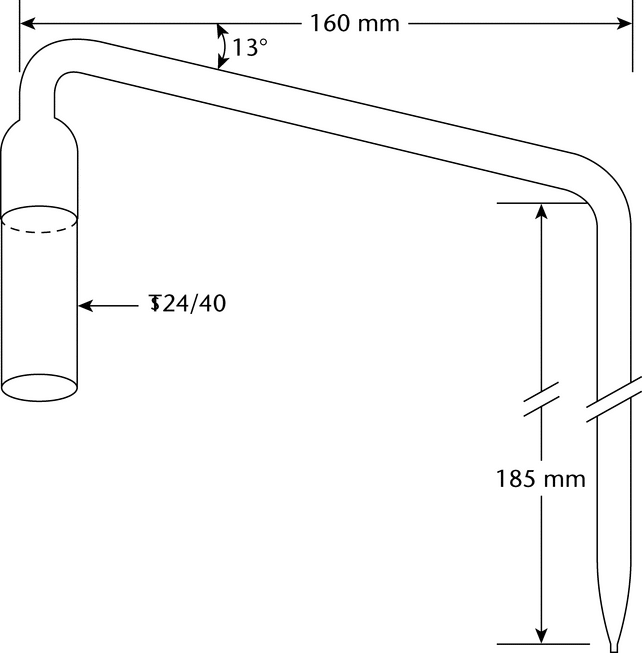
The round-bottom flask, C, is a 1000-mL flask with three 24/40 tapered joints. The gas inlet tube, D, is long enough to permit introduction of the nitrogen to within 2.5 cm of the bottom of the flask. The Allihn condenser, E, has a jacket length of 300 mm. The bubbler, F (see Figure 3), is fabricated from glass according to the dimensions given in Figure 3. The Hydrogen Peroxide Solution is contained in the vessel, G, having an inside diameter of about 2.5 cm and a depth of about 18 cm. Circulate coolant, such as a mixture of water and methanol (4:1) maintained at 5 , to chill the condenser.
, to chill the condenser.
System Suitability Test
Test A—
Using the Potassium Metabisulfite Solution as the standard, proceed as directed for Procedure, except replace the 50.0 g of test substance with 20 mL of Potassium Metabisulfite Solution. Calculate the content, in µg per mL, of sulfur dioxide in the Potassium Metabisulfite Solution taken by the formula:
1000(32.03)VN/VP
in which the factor 1000 converts mg to µg; 32.03 is the milliequivalent weight of sulfur dioxide; V is the volume, in mL, of titrant consumed; N is the normality of the titrant; and VP is the volume, in mL, of Potassium Metabisulfite Solution taken for the test.
Test B—
In a 100-mL conical flask, add 20 mL of 0.02 N iodine solution and 5 mL of 2 N hydrochloric acid. Add 1 mL of starch TS, and titrate with the Potassium Metabisulfite Solution until the first discoloration is observed. Calculate the content, in µg per mL, of sulfur dioxide in the Potassium Metabisulfite Solution by the formula:
1000(32.03)VINI/VP
in which 1000 and 32.03 are defined above; VI is the volume, in mL, of iodine solution used in the test; NI is the normality of the iodine solution; and VP is the volume, in mL, of Potassium Metabisulfite Solution consumed.
The difference between the sulfur dioxide contents obtained from Test A and Test B is not more than 5% of their mean value. Test B shall be performed within 15 minutes after completion of Test A. [Note—This avoids a potential variation of the sulfur dioxide content in the Potassium Metabisulfite Solution when stored at room temperature. ]
Procedure
Position the apparatus in a heating mantle controlled by a power-regulating device. Add 400 mL of water to the flask. Close the stopcock of the separatory funnel, and add 90 mL of 4 N hydrochloric acid to the separatory funnel. Begin the flow of nitrogen at a rate of 200 ± 10 mL per minute. Start the condenser coolant flow. Add 30 mL of Hydrogen Peroxide Solution to the vessel (G). After 15 minutes, remove the separatory funnel, and transfer a mixture of 50.0 g of the test specimen, accurately weighed, and 100 mL of alcohol solution (5 in 100) to the flask. Apply stopcock grease to the outer joint of the separatory funnel, return the separatory funnel to the tapered joint flask, and concomitantly resume the nitrogen flow. Apply headpressure above the hydrochloric acid solution in the separatory funnel with a rubber bulb equipped with a valve. Open the stopcock of the separatory funnel to permit the hydrochloric acid solution to flow into the flask. Continue to maintain sufficient pressure above the hydrochloric acid solution to force it into the flask. [Note—The stopcock may be temporarily closed, if necessary, to increase the pressure. ] To guard against escape of sulfur dioxide into the separatory funnel, close the stopcock before the last few mL of hydrochloric acid drain out. Apply power to the heating mantle sufficient to cause about 85 drops of reflux per minute. After refluxing for 1.75 hours, remove the vessel (G), add 3 drops of methyl red TS, and titrate the contents with 0.01 N sodium hydroxide VS, using a 10-mL buret with an overflow tube and a hose connection to a carbon dioxide-absorbing tube, to a yellow endpoint that persists for at least 20 seconds. Perform a blank determination, and make any necessary correction (see Titrimetry  541
541 ). Calculate the quantity, in µg, of SO2 in each g of the test specimen taken by the formula:
). Calculate the quantity, in µg, of SO2 in each g of the test specimen taken by the formula:
1000(32.03)VN/W
in which the factor 1000 converts mg to µg; 32.03 is the milliequivalent weight of sulfur dioxide; V is the volume, in mL, of titrant consumed; N is the normality of the titrant; and W is the weight, in g, of the test specimen taken.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Hong Wang, Ph.D.
Senior Scientific Liaison 1-301-816-8351 |
(GCCA2010) General Chapters - Chemical Analysis |
USP35–NF30 Page 199
Pharmacopeial Forum: Volume No. 35(2) Page 341