Methodology for the determination of particulate matter in injections is contained in USP  788
788 , which has been harmonized with the European Pharmacopoeia and the Japanese Pharmacopoeia. Sections on instrument standardization for light obscuration and other method details were excluded from the harmonized chapter. Chapter
, which has been harmonized with the European Pharmacopoeia and the Japanese Pharmacopoeia. Sections on instrument standardization for light obscuration and other method details were excluded from the harmonized chapter. Chapter  789
789 Particulate Matter in Ophthalmic Solutions has not been harmonized. Chapter
Particulate Matter in Ophthalmic Solutions has not been harmonized. Chapter  1788
1788 includes important instrument standardization and calibration information applicable to
includes important instrument standardization and calibration information applicable to  788
788 and
and  789
789 ; it also includes recommendations for sample handling, laboratory environment, operator training and general advice applicable to the microscopic method.
; it also includes recommendations for sample handling, laboratory environment, operator training and general advice applicable to the microscopic method.
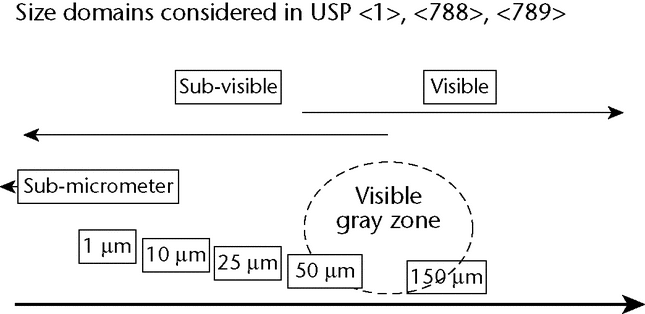
Chapter 1 requires injections to be essentially free from particulate matter that can be observed on visual inspection. The term “essentially free” has been difficult to define because particle detectability is influenced by their number and size, among other factors. The absolute limit of visibility, or detectability, is equivocal and depends upon the test conditions and the nature of the particulate matter. The lower end of the visible range certainly crosses over sub-visible detection capabilities in  788
788 and
and  789
789 . Literature reports visibility extending to 50 µm, 100 µm, and 150 µm size (see References 1 and 2), and membrane assay can isolate and size particles to 1000 µm and larger.
. Literature reports visibility extending to 50 µm, 100 µm, and 150 µm size (see References 1 and 2), and membrane assay can isolate and size particles to 1000 µm and larger.
Chapter  788
788 specifies limits for the sub-visible particulate matter content in injections in two size thresholds. Likewise, Chapter
specifies limits for the sub-visible particulate matter content in injections in two size thresholds. Likewise, Chapter  789
789 establishes particle content expectations for ophthalmic solutions in two (Light Obscuration, or LO) or three (Membrane Microscope, or MM) size thresholds. The tests described in
establishes particle content expectations for ophthalmic solutions in two (Light Obscuration, or LO) or three (Membrane Microscope, or MM) size thresholds. The tests described in  788
788 and
and  789
789 are physical limit tests performed for the purpose of enumerating sub-visible particles [particulate matter] within specific size ranges (see Figure 1).
are physical limit tests performed for the purpose of enumerating sub-visible particles [particulate matter] within specific size ranges (see Figure 1).
Chapter  788
788 states, “Particulate matter consists of mobile undissolved particles, other than gas bubbles, unintentionally present in the solutions.” Using the
states, “Particulate matter consists of mobile undissolved particles, other than gas bubbles, unintentionally present in the solutions.” Using the  788
788 test methods, any semi-solid to solid material (and even immiscible liquids) that trigger LO detector response above a selected size threshold will be tabulated.
test methods, any semi-solid to solid material (and even immiscible liquids) that trigger LO detector response above a selected size threshold will be tabulated.
There are two general categories of particulate matter sources: extrinsic and intrinsic. LO and MM methods will detect and tabulate particles of both categories. Extrinsic material is additive, foreign, and unchanging, and not part of the formulation, package, or assembly process.
Examples of extrinsic material include fibers, cellulosic matter, vegetative matter, corrosion products, paint/coatings, and building materials such as gypsum, concrete, metal, and plastic. Extrinsic particles are additive and generally non-changing over the life of the product, unless by fragmentation, swelling (hydration), or degradation. Fragments of rubber, plastic, metal, and glass are examples of extrinsic particulate matter deposited in the product during assembly or not removed in the container preparation process. However, if these typically extrinsic types have come from the specific package and/or process in a more consistent or chronic manner, then one may consider their presence to be an intrinsic variety, with a similar level of concern.
Intrinsic material is associated with the package, formulation ingredients and process or assembly process. Intrinsic material may also be extraneous material carried by the package or process and insufficiently removed. Intrinsic material may indeed change upon aging, due to concentration change, degradation, and acceleration of reaction.
Intrinsic sources are inherent in the product and process—formulation, package, and commercial assembly steps. Intrinsic sources represent a variety of phenomena yielding unwanted substances, such as: (a) extraction, (b) leaching, (c) degradation of ingredient (active or excipient), (d) change of ingredient by precipitation/salt form/crystalline form, (e) change of package physical integrity, (f) change of impurity level, (g) change of micellar association, (h) oligamerization, and (i) package- and process-related materials not removed during product assembly. Combinations of all of the above and physical phenomena such as aggregation, sedimentation, and coalescence by matrix (oils, semi-solids) may bring smaller particles (<10 µm) into the detection zone of the test method ( 10 µm). Intrinsic sources of detectable particulate matter are of great concern, since the substance may be present, however, not evident until particles form over time, even long after lot release.
10 µm). Intrinsic sources of detectable particulate matter are of great concern, since the substance may be present, however, not evident until particles form over time, even long after lot release.
The intrinsic categorization should be recognized as different from inherent formulation character. Solution properties such as a slight haze or faint coloration of high concentration formulae and protein formulations are typical examples of an inherent characteristic of the product fluid, and while the condition may cause difficulties in inspection or LO assay, are not particle-related.
Certain solution formulations may not be easily analyzed by LO. The LO method may encounter problems with a product that does not have clarity and a viscosity approximating those of water. Further, formulation characteristics such as color, high viscosity or inherent formulation properties, such as shear-induced changes, may generate erroneous LO data. Similarly, products that produce air or gas bubbles when drawn into the LO sensor, such as bicarbonate-buffered formulations may generate erroneous data. For these product types the MM method may have to be used. Documentation demonstrating that the LO procedure is incapable of testing the test article or produces invalid results may aid regulatory filing strategy. It is expected that most test articles will meet the requirements on the basis of the LO test alone; however, it may be necessary to assay some test articles by the LO method followed by the MM method in order to reach a conclusion.
There may be a desire to test lower volumes of certain products, due to limited sample, high product cost, low container volume, or due to special fluid delivery characteristics. Examples include biopharmaceuticals, low-volume parenteral and ophthalmic products and formulations in novel packages intended for specific medical targets. The expectation is limits compliance for these products; however, one may employ methods validated by the manufacturer to demonstrate conformance with the test limits. Special low-volume “sippers” for LO sampling and the pooling of multiple containers may be necessary for these package presentations. Consider this example: a low-volume (100 µL) product is packaged in a pre-filled sterile syringe. The nature of the package allows simple delivery of the solution product and may be used for direct sampling, but the 100 µL volume precludes the pooling of the larger volumes (~25 mL) for the LO method. Direct sampling to a small membrane for microscopical counting and evaluating single and pooled package particle content may be the optimal means to collect data. Also in this example, careful statistical evaluation of the batch population using small sample volumes (but not doses) will be necessary to validate product acceptability.
LIGHT OBSCURATION PARTICLE COUNT TEST
Test Apparatus
The apparatus is a liquid-borne particle counting system that uses a light-obscuration sensor with a suitable sample-feeding device to deliver controlled aliquots of sample for analysis. Suspended particles in the sample fluid flowing between a light source and sensor produce changes in signal that are correlated to particle dimension. Due to the nature of the detection and counting system, air bubbles and immiscible liquids may block sufficient light to be recorded along with the target suspended particles. These artifacts must be diminished through proper preparation techniques. Solutions with excessive immiscible liquids may not be amenable to LO analysis. A variety of suitable devices of this type are commercially available. It is the responsibility of those performing the test to ensure that the operating parameters of the instrumentation are appropriate to the required accuracy and precision of the test result, the artifacts and interferences inherent in certain products and with certain methods of preparation are eliminated or accommodated. An example is a protein formulation that may form shear-induced semi-solids due to mixing and counted as “particles.” Adequate training must be provided for those responsible for the technical performance of the test.
It is important to note that for Pharmacopeial applications the ultimate goal is that the particle counter reproducibly size and count particles present in the material under investigation. The instruments available range from systems where calibration and other components of standardization must be carried out by manual procedures to sophisticated systems incorporating hardware- and software-based functions for the standardization procedures. Thus, it is not possible to specify exact methods to be followed for standardization of the instrument, and it is necessary to emphasize the required end result of a standardization procedure rather than a specific method for obtaining this result. This section is intended to emphasize the criteria that must be met by a system rather than specific methods to be used in their determination. It is the responsibility of the user to apply the various methods of standardization applicable to a specific instrument. Critical operational criteria consist of the following.
Sensor Concentration Limits—
Use an instrument that has a concentration limit (the maximum number of particles per mL) identified by the manufacturer that is greater than the concentration of particles in the test specimen to be counted. The vendor-certified concentration limit for a sensor is specified as that count level at which coincidence counts due to the simultaneous presence of two or more particles in the sensor view volume compose less than 10% of the counts collected for 10-µm particles.
Sensor Dynamic Range—
The dynamic range of the instrument used (range of sizes of particles that can be accurately sized and counted) must include the smallest particle size to be enumerated in the products.
Instrument Standardization Tests
The following discussion of instrument standardization emphasizes performance criteria rather than specific methods for calibrating or standardizing a given instrument system. This approach is particularly evident in the description of calibration, where allowance must be made for manual methods as well as those based on firmware, software, or the use of electronic testing instruments. Appropriate instrument qualification is essential to performance of the test according to requirements. Since different brands of instruments may be used in the test, the user is responsible for ensuring that the counter used is operated according to the manufacturer's specific instruction; the principles to be followed to ensure that instruments operate within acceptable ranges are defined below. The following information for instrument standardization helps ensure that the sample volume accuracy, sample flow rate, particle size response curve, sensor resolution, and count accuracy are appropriate to performance of the test. Conduct these procedures at intervals of not more than six months.
sample volume accuracy
Since the particle count from a sample aliquot varies directly with the volume of fluid sampled, it is important that the sampling accuracy is known to be within a certain range. For a sample volume determination, determine the dead (tare) volume in the sample feeder with particle-free water.1 Transfer a volume of particle-free water that is greater than the sample volume to a container, and weigh. Using the sample feeding device, withdraw a volume that is appropriate for the specific sampler, and again weigh the container. Determine the sample volume by subtracting the tare volume from the combined sample plus tare volumes. Verify that the value obtained is within 5% of the appropriate sample volume for the test. Alternatively, the sample volume may be determined using a suitable Class A graduated cylinder (see Volumetric Apparatus  31
31 ). [note—Instruments of this type require a variable tare volume. This is the amount of sample withdrawn before counting. This volume may be determined for syringe-operated samplers by setting the sample volume to zero and initiating sampling, so that the only volume of solution drawn is the tare. Subtract the tare volume from the total volume of solution drawn in the sampling cycle to determine the sample volume. ]
). [note—Instruments of this type require a variable tare volume. This is the amount of sample withdrawn before counting. This volume may be determined for syringe-operated samplers by setting the sample volume to zero and initiating sampling, so that the only volume of solution drawn is the tare. Subtract the tare volume from the total volume of solution drawn in the sampling cycle to determine the sample volume. ]
sample flow rate
Verify that the flow rate is within the manufacturer's specifications for the sensor used. This may be accomplished by using a calibrated stopwatch to measure the time required for the instrument to withdraw and count a specific sample volume (i.e., the time between beginning and ending of the count cycle as denoted by instrument indicator lights or other means). Sensors may be operated accurately over a range of flow rates. Perform the Test Procedure below at the same flow rate as that selected for calibration of the instrument.
calibration
USP  788
788 specifies the use of dispersions of spherical particles of known sizes between 10 µm and 25 µm in particle-free water. More options follow:
specifies the use of dispersions of spherical particles of known sizes between 10 µm and 25 µm in particle-free water. More options follow:
Manual Method—
Calibrate the instrument with a minimum of three calibrators, such as near-mono-size polystyrene spheres having diameters of about 10, 15, and 25 µm, in an aqueous particle-free vehicle. The calibrator spheres must have a mean diameter of within 5% of the nominal diameters and be standardized against materials traceable to NIST standard reference materials.2 The total number of spheres counted must be within the sensor's concentration limit. Prepare suspensions of the calibrator spheres in water at a concentration of 1000 to 5000 particles per mL, and determine the channel setting that corresponds to the highest count setting for the sphere distribution. This is determined by using the highest count threshold setting to split the distribution into two bins containing equal numbers of counts, with the instrument set in the differential count mode (moving window half-count method). Use only the central portion of the distribution in this calculation to avoid including asymmetrical portions of the peak. The portion of the distribution, which must be divided equally, is the count window. The window is bounded by threshold settings that will define a threshold voltage window of ±20% around the mean diameter of the test spheres. The window is intended to include all single spheres, taking into account the standard deviation of the spheres and the sensor resolution, while excluding noise and aggregates of spheres. The value of 20% was chosen on the basis of the worst-case sensor resolution of 10% and the worst-case standard deviation of the spheres of 10%. Since the thresholds are proportional to the cross-sectional area of the spheres (and all particles) rather than the diameter, the lower and upper voltage settings are determined by the equations:
VL = 0.64VS
in which VL is the lower voltage setting, and VS is the voltage at the peak center, and
VU = 1.44VS
in which VU is the upper voltage setting. Once the center peak thresholds are determined, use these thresholds for the standards to create a regression of log voltage versus log particle size, from which the instrument settings for the 10- and 25-µm sizes can be determined.
Automated Method—
The calibration (size response) curve may be determined for the instrument-sensor system by the use of validated software routines offered by instrument vendors; these may be included as part of the instrument software or used in conjunction with a microcomputer interfaced to the counter. The use of these automated methods is appropriate if the vendor supplies written certification that the software provides a response curve equivalent to that attained by the manual method and if the automated calibration is validated as necessary by the user.
Electronic Method—
Using a multichannel peak height analyzer, determine the center channel of the particle counter pulse response for each standard suspension. This peak voltage setting becomes the threshold used for calculation of the voltage response curve for the instrument. The standard suspensions used for the calibration are run in order, and median pulse voltages for each are determined. These thresholds are then used to generate the size response curve manually or via software routines. The thresholds determined from the multichannel analyzer data are then transferred to the counter to complete the calibration.
sensor resolution
The particle size resolution of the instrumental particle counter is dependent upon the sensor used and may vary with individual sensors of the same model. Determine the resolution of the particle counter for 10-µm particles, using the 10-µm calibrator spheres. The relative standard deviation of the size distribution of the standard particles used is not more than 5%. Acceptable methods of determining particle size resolution are (1) manual determination of the amount of peak broadening due to instrument response; (2) using an electronic method of measuring and sorting particle sensor voltage output with a multichannel analyzer; and (3) automated methods.
Manual Method—
Adjust the particle counter to operate in the cumulative mode or total count mode. Refer to the calibration curve obtained earlier, and determine the threshold voltage for the 10-µm spheres. Adjust 3 channels of the counter to be used in the calibration procedure as follows:
Channel 1 is set for 90% of the threshold voltage.
Channel 2 is set for the threshold voltage.
Channel 3 is set for 110% of the threshold voltage.
Draw a sample through the sensor, observing the count in Channel 2. When the particle count in that channel has reached approximately 1000, stop counting, and observe the counts in Channels 1 and 3. Check to see if the Channel 1 count and Channel 3 count are 1.68 ± 10% and 0.32 ± 10%, respectively, of the count in Channel 2. If not, adjust Channel 1 and Channel 3 thresholds to meet these criteria. When these criteria have been satisfied, draw a sample of suspension through the counter until the counts in Channel 2 have reached approximately 10,000, or until an appropriate volume (e.g., 10 mL) of the sphere suspension has been counted. Verify that Channel 1 and Channel 3 counts are 1.68 ± 3% and 0.32 ± 3%, respectively, of the count in Channel 2. Record the particle size for the thresholds just determined for Channels 1, 2, and 3. Subtract the particle size for Channel 2 from the size for Channel 3. Subtract the particle size for Channel 1 from the size for Channel 2. The values so determined are the observed standard deviations on the positive and negative side of the mean count for the 10-µm standard. One commonly used method for calculating the percentage of resolution of the sensor is the following:
% resolution = (100/D) × [(SObs)2  (SStd)2]½
(SStd)2]½
in which SObs is the highest observed standard deviation determined for the sphere standard; SStd is the supplier’s reported standard deviation for the spheres; and D is the diameter, in µm, of the spheres as specified by the supplier. The resolution is not more than 10%.
Automated Method—
Software that allows for the automated determination of sensor resolution is available for some counters. This software may be included in the instrument or used in conjunction with a microcomputer interfaced to the counter. The use of these automated methods is appropriate if the vendor supplies written certification that the software provides a resolution determination equivalent to the manual method and if the automated resolution determination is validated as necessary by the user.
Electronic Method—
Record the voltage output distribution of the particle sensor, using a multichannel analyzer while sampling a suspension of the 10-µm particle size standard. To determine resolution, move the cursor of the multichannel analyzer up and down the electric potential scale from the median pulse voltage to identify a channel on each side of the 10-µm peak that has approximately 61% of the counts observed in the center channel. Use of the counter size response curve to convert the mV values of these two channels to particle sizes provides the particle size at within one standard deviation of the 10-µm standard. Use these values to calculate the resolution as described under Manual Method.
particle counting accuracy—system suitability
Determine the particle counting accuracy of the instrument, using Method 1 (for sensors requiring the moving window half-count (MWHC) method for calibration), Method 2 (for multichannel sensors), or Method 3 for any instrument (manual comparison to membrane microscope method).
Method 1—MWHC Instruments
Procedure—
Prepare the suspension and blank using the USP Particle Count RS. With the instrument set to count in the cumulative (total) mode, collect counts at settings of greater than or equal to 10 µm and greater than or equal to 15 µm. Prepare the blank and suspension sample in the same manner. Degas the mixture by one of three means: by sonication (at 80 to 120 watts) for about 30 seconds, by allowing to stand, or by vacuum. Gently stir the contents by hand-swirling or by mechanical means, taking care not to introduce air bubbles or contamination. Stir continuously throughout the analysis. Withdraw directly from the container three consecutive volumes. Historically, these have been volumes of not less than 5 mL each, due to instrument limitations and the desire to maximize sample volume. However, where desired, volumes may be utilized that meet the standardization criteria and address the sensitivities of the formulation. Obtain the particle counts, and discard the data from the first portion. [note—Complete the procedure within five minutes. ] Repeat the procedure, using the suspension in place of the blank. From the averages of the counts resulting from the analysis of the two portions of the suspension at greater than or equal to 10 µm and from the analysis of the two portions of the blank at greater than or equal to 10 µm, calculate the number of particles in each mL by the formula:
(PS – PB)/V
in which PS is the average particle count obtained from the suspension; PB is the average particle count obtained from the blank; and V is the average volume, in mL, of the 4 portions tested. Repeat the calculations, using the results obtained at the setting of not less than 15 µm.
Interpretation—
The MWHC instrument meets the requirements for Particle Counting Accuracy if the count obtained at  10 µm and the ratio of the counts obtained at greater than or equal to 10 µm to those obtained at greater than or equal to 15 µm conform to the values that accompany the USP Particle Count RS. If the instrument does not meet the requirements for Particle Counting Accuracy, and adequate test volumes remain, repeat the procedure with them; if insufficient volumes remain, prepare new suspension and blank, and then repeat the procedure. If the results of the second test are within the limits given above, the instrument meets the requirements of the test for Particle Counting Accuracy. If on the second attempt the system does not meet the requirements of the test, determine and correct the source of the failures, and retest the instrument.
10 µm and the ratio of the counts obtained at greater than or equal to 10 µm to those obtained at greater than or equal to 15 µm conform to the values that accompany the USP Particle Count RS. If the instrument does not meet the requirements for Particle Counting Accuracy, and adequate test volumes remain, repeat the procedure with them; if insufficient volumes remain, prepare new suspension and blank, and then repeat the procedure. If the results of the second test are within the limits given above, the instrument meets the requirements of the test for Particle Counting Accuracy. If on the second attempt the system does not meet the requirements of the test, determine and correct the source of the failures, and retest the instrument.
Method 2—Multichannel Instruments
Procedure—
Use one of three standards: (1) a dilution of the USP Particle Count Reference Standard (USP PCRS); (2) commercial preparation of standard calibrator spheres of nominal diameter 15 to 30 µm in a suspension containing between 50 and 200 particles per mL, certified by the manufacturer; or (3) a laboratory-prepared suspension of standard calibrator spheres having a nominal diameter of 15 to 30 µm, containing between 50 and 200 particles per mL. Use of non-USP standards 2 and 3 is acceptable when they are compliant with USP standardization criteria: five successive counts are not more than ±10% of stated size.
Degas the suspension by one of three means: by sonication (at 80 to 120 watts) for about 30 seconds, by allowing to stand, or by vacuum. Gently stir the contents by hand-swirling or by mechanical means, taking care not to introduce air bubbles or contamination. Stir continuously throughout the analysis, and perform five counts on 5-mL volumes of the suspension, using the particle counter 10-µm size threshold. Obtain the mean cumulative particle count per mL.
Interpretation—
The instrument meets the requirements for Particle Counting Accuracy if the count obtained at greater than or equal to 10 µm conforms to the values that accompany the USP Particle Count RS. If the instrument does not meet the requirements for Particle Counting Accuracy, repeat the procedure. If the results of the second test are within the limits given above, the instrument meets the requirements of the test for Particle Counting Accuracy. If on the second attempt the system does not meet the requirements of the test, determine and correct the source of the failures, and retest the instrument.
Method 3—Alternate Manual Method
Procedure—
Prepare a suspension of standard calibrator spheres having a nominal diameter of 15 to 30 µm, containing between 50 and 200 particles per mL. Degas the suspension by one of three means: by sonication (at 80 to 120 watts) for about 30 seconds, by allowing to stand, or by vacuum. Gently stir the contents by hand-swirling or by mechanical means, taking care not to introduce air bubbles or contamination. Stir continuously throughout the analysis and perform five counts on 5-mL volumes of the suspension, using the particle counter 10-µm size threshold. Obtain the mean cumulative particle count per mL. Pipette a volume of this suspension containing 250 to 500 particles into a filter funnel prepared as described for Microscope Particle Count Test, Filtration Apparatus, below. After drying the membrane, count the total number of standard spheres collected on the membrane filter. This count should be within 20% of the mean instrumental count per mL for the suspension.
Test Environment
Specimens must be cleaned to the extent that the level of particles added by testing has a negligible effect on the outcome of the test.
Cleanse glassware, closures, and other required equipment, preferably by immersing and cleaning the items using warm, nonionic detergent solution. Rinse in flowing tap water, and then rinse again in flowing filtered water. Organic solvents may also be used to facilitate cleaning. [note—These steps describe one way to clean equipment; alternatively, particulate-free equipment may be obtained from a suitable vendor. ] Preferably, the test specimen, glassware, closures, and other required equipment are then finally rinsed with filtered water, using a hand-held pressure nozzle with final filter or other appropriate filtered water source within an environment protected by high-efficiency particulate air (HEPA) filters. While conducting the assay, non-shedding garments and powder-free gloves are worn within the HEPA environment. Perform the test in an environment that does not contribute any significant amount of particulate matter.
To collect blank counts, use a cleaned vessel of the type and volume representative of that to be used in the test. Place a 50-mL or more volume of filtered water in the vessel, and agitate the water sample in the cleaned glassware by inversion or swirling. [note—A smaller volume, consistent with the article to be counted, can be used. ] Degas by the same method to be used for the product samples, by one of three means; sonication (at 80 to 120 watts) for about 30 seconds, by vacuum, or by allowing to stand. Swirl the vessel containing the water sample by hand or agitate by mechanical means to suspend particles.
As described in  788
788 : Determine the particulate matter in 5 samples of filtered water, each of 5 ml. If the number of particles of 10 µm or greater size exceeds 25 for the combined 25 ml (NMT 1/mL), the precautions taken for the tests are not sufficient.
: Determine the particulate matter in 5 samples of filtered water, each of 5 ml. If the number of particles of 10 µm or greater size exceeds 25 for the combined 25 ml (NMT 1/mL), the precautions taken for the tests are not sufficient.
It is recommended that when utilizing the test for the  789
789 method, the blank test should be considered failed, if in addition, the number of particles of 25 µm or greater in size exceeds 3.
method, the blank test should be considered failed, if in addition, the number of particles of 25 µm or greater in size exceeds 3.
Test Procedure
test preparation
Prepare the test specimens in the following sequence. Outside of the unidirectional airflow cabinet to be used for the test, remove outer closures, sealing bands, but not the sealing closure. If shedding is noted to be an issue, remove or tape over the product labels as well. Place the samples in the test cabinet, and rinse the exteriors of the containers with filtered water as directed under Test Environment. Protect the containers from environmental contamination until analyzed. After proper mixing, open and withdraw, pour or otherwise sample the contents of the containers under test in a manner least likely to generate particles that could enter the test specimen. Contents of containers with removable stoppers may be poured out directly after removing the closures. Sampling devices having a needle to penetrate the unit closure may also be employed. Products packaged in flexible plastic containers may be sampled by cutting the medication or administration port tube or a corner from the unit with a suitably cleaned razor blade or scissors. Dry or lyophilized products may be constituted using their internal diluent, by removing the closure to add supplied product diluent or by injecting filtered water via hypodermic syringe. If test specimens are to be pooled, remove the closure and empty the contents into a clean container.
number of test specimens
USP  788
788 provides the sampling plan according to product volume. For all products, regardless of volume, comprehensive experience regarding the integrity and consistency of the batch is gained throughout development, allowing the proper sampling plans to be applied in commercial production that ensure sample selection is representative of batch quality. All batches must have sampling plans that accommodate desired statistical measures of batch quality and facilitate process control.
provides the sampling plan according to product volume. For all products, regardless of volume, comprehensive experience regarding the integrity and consistency of the batch is gained throughout development, allowing the proper sampling plans to be applied in commercial production that ensure sample selection is representative of batch quality. All batches must have sampling plans that accommodate desired statistical measures of batch quality and facilitate process control.
product determination
Depending upon the dosage form being tested, proceed as directed under the appropriate category below.
Liquid Preparations—
Volume in Container Less Than 25 mL—
Prepare the containers as directed under Test Preparation. Mix and suspend the particulate matter in each unit by inverting the unit 20 times. [note—Because of the small volume of some products, it may be necessary to agitate the solution more vigorously to suspend the particles properly. ] Open and combine the contents of 10 or more units in a cleaned container to obtain a volume of not less than 25 mL. Degas the pooled solution by one of three means: sonication for about 30 seconds, or by vacuum, or by allowing the solution to stand.
Gently stir the contents of the container by hand-swirling or by mechanical means, taking care not to introduce air bubbles or contamination. Remove four portions, that conform to the volumes utilized in the IST, and count the number of particles equal to or greater than 10 µm and 25 µm. Disregard the result obtained for the first portion. [note—For low-volume products, a pool of 15 or more units may be necessary to achieve a pool volume sufficient for four 5-mL sample aliquots. Smaller sample aliquots (i.e., less than 5 mL) can be used if the assay result obtained with the smaller aliquots is validated to give an assessment of batch suitability equivalent to that obtained with the 5-mL aliquots specified above. ]
Volume in Container 25 mL or More—
Prepare the containers as directed under Test Preparation. Mix and suspend the particulate matter in each unit by inverting the unit 20 times prior to opening the container for degassing. Degas the solution by one of three means: by sonication for about 30 seconds, or by vacuum, or by allowing the solution to stand. When sampling, ensure that the counter probe can be inserted into the middle of the solution. Gently stir the contents of the unit by hand-swirling or by mechanical means. Remove four portions, each of not less than 5 mL, and count the number of particles equal to or greater than 10 µm and 25 µm. Disregard the result obtained for the first portion.
Dry or Lyophilized Preparations—
Prepare the containers as directed under Test Preparation. Open each container, taking care not to contaminate the opening or cover. Constitute as directed by the labeling, according to the Test Preparation. Alternately, depending on the experiment, use:
Replace the closure, and manually agitate the container sufficiently to ensure dissolution of the drug. [note—For some dry or lyophilized products, it may be necessary to let the containers stand for a suitable interval, and then agitate again to effect complete dissolution. ] After the drug in the constituted sample is completely dissolved, degas the solution by sonication for about 30 seconds, or by exposing to vacuum, or by allowing the solution to stand. When sampling, ensure that the counter draw or sipping probe can be inserted into the middle of the solution. Gently stir the contents of the unit by hand-swirling or by mechanical means to mix and suspend any particulate matter. Proceed as directed for the appropriate unit volume under Liquid Preparations, and analyze by withdrawing a minimum of four portions, each of not less than 5 mL, and count the number of particles equal to or greater than 10 µm and 25 µm. Disregard the result obtained for the first portion.
- filtered water or
- an appropriate laboratory-filtered diluent if suitable.
Products Packaged with Dual Compartments Constructed to Hold the Drug Product and a Solvent in Separate Compartments—
Prepare the units to be tested as directed under Test Preparation and according to product insert directions. Mix each unit as directed in the labeling, activating and agitating so as to ensure thorough mixing of the separate components and drug dissolution. Open and degas the units or pooled specimen to be tested by one of three means: sonication, or by vacuum, or by allowing the solution to stand. Proceed as directed for the appropriate unit volume under Liquid Preparations, mix and suspend the particulate matter present in each unit by inversion or swirling or by mechanical means and analyze by withdrawing a minimum of four portions, each of not less than 5 mL, and count the number of particles equal to or greater than 10 µm and 25 µm. Disregard the result obtained for the first portion.
Products Labeled “Pharmacy Bulk Package Not for Direct Infusion”—
Proceed as directed for Liquid Preparations where the volume is 25 mL or more. Calculate the test result on a portion that is equivalent to the maximum dose given in the labeling. For example, if the total bulk package volume is 100 mL and the maximum dose volume is 10 mL, then the average LO particle count per mL would be multiplied by 10 to obtain the test result based on the 10-mL maximum dose. [Note—For the calculations of test results, consider this maximum dose portion to be the equivalent of the contents of one full container. ]
LO Calculations
Note that the particle limits must be reported as all particles  10 µm and all particles
10 µm and all particles  25 µm. If the instrument has been configured to count in differential bins, such as
25 µm. If the instrument has been configured to count in differential bins, such as  10–25 µm,
10–25 µm,  25–50 µm,
25–50 µm,  50 µm, etc., all bins
50 µm, etc., all bins  10 µm must be added to yield total
10 µm must be added to yield total  10 µm count; all bins
10 µm count; all bins  25 µm need to be added to yield total count
25 µm need to be added to yield total count  25 µm.
25 µm.
For example, the analyst has counted the test samples in eight bins: a)  10–15 µm, b)
10–15 µm, b)  15 µm–25 µm, c)
15 µm–25 µm, c)  25 µm–40 µm, d)
25 µm–40 µm, d)  40 µm–75 µm e)
40 µm–75 µm e)  75 µm–100 µm and f)
75 µm–100 µm and f)  100 µm. They would then calculate PS
100 µm. They would then calculate PS 10 as:
10 as:
PS 10 = P
10 = P 10–15 µm + P
10–15 µm + P 15–25 µm + P
15–25 µm + P 25–40 µm + P
25–40 µm + P 40–75 µm + P
40–75 µm + P 75–100 µm + P
75–100 µm + P 100 µm
100 µm
Pooled Samples—
Average the counts from the two or more aliquot portions analyzed. Calculate the number of particles in each container by the formulae:
 25 µm analyzed. VTis the volume of pooled sample, in mL; VA is the volume, in mL, of each portion analyzed; and n is the number of containers pooled.
25 µm analyzed. VTis the volume of pooled sample, in mL; VA is the volume, in mL, of each portion analyzed; and n is the number of containers pooled.
PS10VT/VAn
PS25VT/VAn
in which PS10 is the average particle count per threshold obtained from all portions analyzed and PS25 is the average particle count per threshold obtained from all portions
Individual Samples—
Average the counts obtained for the 5-mL or greater aliquot portions from each separate unit analyzed, and calculate the number of particles in each container by the formulae:
 25 µm analyzed. V is the volume, in mL, of the tested unit; and VA is the volume, in mL, of each portion analyzed.
25 µm analyzed. V is the volume, in mL, of the tested unit; and VA is the volume, in mL, of each portion analyzed.
PS10V/VA
PS25V/VA
in which PS10 is the average particle count per threshold obtained from all portions analyzed; and PS25 is the average particle count per threshold obtained from all portions
Individual Unit Samples—
Average the counts obtained for the two or more 5-mL aliquot portions taken from the solution unit. Calculate the number of particles in each mL of product solution taken by the formulae:
 25 µm analyzed. V is the volume, in mL, of the portion taken.
25 µm analyzed. V is the volume, in mL, of the portion taken.
PS10/V
PS25/V
in which PS10 is the average particle count per threshold obtained from all portions analyzed and PS25 is the average particle count per threshold obtained from all portions For all types of product, if the tested material has been diluted to decrease the viscosity, the dilution factor must be accounted for in the calculation of the final test result. For all test results, the particle count  10 µm represents all threshold bin counts.
10 µm represents all threshold bin counts.
MEMBRANE MICROSCOPE PARTICLE COUNT TEST
The microscope particulate matter test may be applied to both large-volume and small-volume parenteral injections and to ophthalmic solution products as well. This test enumerates essentially solid3 particulate matter  10 µm in these products, after collection, rinsing and drying on a micro-porous membrane filter. Since a wide range of test aliquots may be utilized, particle counts may be determined on a per-volume or per-container basis without dilution or extrapolation.
10 µm in these products, after collection, rinsing and drying on a micro-porous membrane filter. Since a wide range of test aliquots may be utilized, particle counts may be determined on a per-volume or per-container basis without dilution or extrapolation.
In the performance of the membrane microscope assay, one estimates the size of retained solids viewed at 100× magnification, tabulating them into specific size categories. In this process, one may encounter materials on the membrane surface that do not appear solid or substantial, showing little or no surface relief such as a “stain” or discontinuity on the membrane. Chapter  788
788 advises not to attempt to size or enumerate such semi-solid particles, due to historical comment from LVP terminal sterilization manufacturers that encountered stain-like brown residues after heat sterilization of Dextrose solutions. However, if not sampling a carbohydrate solution or similarly-performing formulation, recognizing the presence of such material is adds a measure of formulation robustness. Consistent evidence of such materials may be indication that further development research is warranted to understand their content. The nature of these materials and subsequent decision to count or investigate must be based upon product formulation experience. Interpretation of microscopical enumeration may be aided by testing a sample of the solution by the LO particle count or a validated, alternate method.
advises not to attempt to size or enumerate such semi-solid particles, due to historical comment from LVP terminal sterilization manufacturers that encountered stain-like brown residues after heat sterilization of Dextrose solutions. However, if not sampling a carbohydrate solution or similarly-performing formulation, recognizing the presence of such material is adds a measure of formulation robustness. Consistent evidence of such materials may be indication that further development research is warranted to understand their content. The nature of these materials and subsequent decision to count or investigate must be based upon product formulation experience. Interpretation of microscopical enumeration may be aided by testing a sample of the solution by the LO particle count or a validated, alternate method.
The Test Apparatus is described in  788
788 . Additional comments are:
. Additional comments are:
- Use a compound binocular microscope that corrects for changes in interpupillary distance by maintaining a constant tube length.
- The objective must be of 10× nominal magnification, a planar achromat or better in quality, with a minimum 0.25 numerical aperture.
- The objective must be compatible with an episcopic illuminator attachment.
- The eyepieces must be matched. In addition, one eyepiece must be designed to accept and focus an eyepiece graticule. The microscope must have a mechanical stage capable of holding and traversing the entire filtration area of a 25-mm or 47-mm membrane filter.
- Two illuminators are required. Both illuminators must be of sufficient output to provide a bright and even source of illumination and may be equipped with blue daylight filters to decrease operator fatigue during use.
-
The USP graticule as described in
 788
788 is used.
is used.
Stage Micrometer—
Graduated in 10-µm increments, utilized each day-of-use. For initial calibration, utilize a stage micrometer that is certified by NIST to verify the USP graticule installation. Thereafter, for daily calibration/verification, one may utilize a commercial stage micrometer graduated in 10-µm increments to verify proper setup.
Filtration Apparatus—
Use a filter funnel suitable for the volume to be tested, generally having an inner diameter of about 16 mm for 25-mm membranes or about 37 mm for 47-mm membranes. The funnel is made of plastic, glass, or stainless steel. Use a filter support made of stainless steel screen or sintered glass as the filtration diffuser. A solvent dispenser capable of delivering solvents filtered through a membrane filter at a range of pressures from 10 psi to 80 psi.
Membranes—
As described by  788
788 ; however, finer pore size selections will have smoother surfaces, facilitating the microscopical examination; however, may impede more viscous sample fluid during the assay.
; however, finer pore size selections will have smoother surfaces, facilitating the microscopical examination; however, may impede more viscous sample fluid during the assay.
Test Environment
Following discussion is recommended operational detail to enhance the conductance of the MM assay.
It is ideal to use two unidirectional airflow hood (UAFH) or other unidirectional airflow enclosures, one for “wet” sample preparations, and the other an enclosure for the microscope counting phase. The UAFH having a capacity sufficient to envelop the area in which the analysis is prepared. The UAFH provides HEPA-filtered air which typically contains not more than 100 particles (0.5 µm or larger) per cubic foot. A blank determination is necessary at the beginning of each test sequence to verify minimal contribution from the background, equipment and personnel operations. What is the definition of a test sequence? Should it be one per shift, one per product family, one per series of filtrations (manifold) or one per sample? Any of these definitions may be suitable, dependant upon the operational needs of the lab system. The ability to clean glassware between samples, the number of different products being run, and the volume of samples through the lab will determine the appropriate control. However, consider the blank to be a system suitability check, and if it fails, all samples run prior to it up to the previous blank, are suspect.
To determine the blank count, duplicate the sample preparation process, in regard to the apparatus and membrane types. Assemble a clean filtration apparatus with a fresh membrane, rinse the interior with filtered water to drain, then deliver a 50-mL or more volume of filtered water to the filtration funnel while applying vacuum, and draw the entire volume of water through the membrane filter. Remove the membrane from the filter funnel base, and place onto a holding device as will be used for test specimens; typically atop a strip of double-sided tape on a microscope slide or in a commercial membrane holder or Petri dish. After allowing the membrane to dry (it must be counted dry), examine the entire filtration area microscopically at a magnification of 100×. If not more than 20 particles  10 µm and not more than 5 particles
10 µm and not more than 5 particles  25 µm or larger are present within the filtration area, the background particle level is sufficiently low for performance of the microscope assay for
25 µm or larger are present within the filtration area, the background particle level is sufficiently low for performance of the microscope assay for  788
788 . If particle load exceeds these limits, repeat the procedure.
. If particle load exceeds these limits, repeat the procedure.
There is value in further limiting the background for both  788
788 and
and  789
789 testing in regard to good laboratory practice, and more specifically in regard to the
testing in regard to good laboratory practice, and more specifically in regard to the  789
789
 25 µm and
25 µm and  50 µm limits, which may be considered more restrictive than injectable limits in consideration of total particle content allowed for the (usually) small unit volumes. Compare total particle load for a small SVI, small LVI versus a 5-mL ophthalmic product in Table 1.
50 µm limits, which may be considered more restrictive than injectable limits in consideration of total particle content allowed for the (usually) small unit volumes. Compare total particle load for a small SVI, small LVI versus a 5-mL ophthalmic product in Table 1.
Table 1. Comparison of Total At-Limit Load for Selected Products.
| Size
Limit |
Blank
Count |
SVI,
5 mL |
LVI,
125 mL |
Ophthalmic
Product, 5 mL |
|---|---|---|---|---|
| 20 | 3000 particles |
1500 particles |
250 particles |
|
| 5 | 300 particles |
250 particles |
25 particles |
|
| not defined |
N/A | N/A | 10 particles |
Therefore, in smaller volume  789
789 applications and in low-particle count injectable product, the laboratory should strive for consistent and lower blank counts such as NMT 5
applications and in low-particle count injectable product, the laboratory should strive for consistent and lower blank counts such as NMT 5  10 µm, NMT 1
10 µm, NMT 1  25 µm and none
25 µm and none  50 µm per blank.
50 µm per blank.
Throughout the operational procedure (in the HEPA environment), it is preferable to use powder-free gloves, and low-shedding clothing. Prior to conducting the test, clean the work surfaces of the unidirectional flow enclosure with an appropriate filtered solvent. Glassware and equipment should be rinsed successively with a warm, residue-free solution of detergent, hot water, filtered distilled or deionized water, and isopropyl alcohol. [Note—Prior to use, pass the distilled or deionized water and the isopropyl alcohol through membrane filters having a nominal pore size of 0.2-µm or finer. ] Perform the rinsing in the unidirectional airflow enclosure. Allow the glassware and filtration apparatus to dry in the unidirectional airflow enclosure, upstream of all other operations. Preferably, the enclosure is located in a separate room that is supplied with filtered air-conditioned air and maintained under positive pressure with respect to the surrounding areas.
microscope preparation
The microscope optical alignment and illumination are critical for success of this method. Although it is not difficult to differentiate a 10 µm from a 25 µm particle at 100× with reflected light, the decision regarding the boundary at each size category will be difficult with poor equipment, maintenance or optical alignment. Also operator fatigue is caused by poor microscope alignment. We will have to make decisions such as “is this particle 9 µm or 11 µm?” and “is that particle 24 µm or 26 µm?” Optimized system resolution, that is, the ability to discern discrete points of minimal separation, relies upon good optical systems, aligned well. Factors including instrument cleanliness, resolution, e.g., objective N.A.,4 focus of both eyepieces and the graticule will play significant roles in attaining best images. In consideration of optimizing the use of the binocular compound microscope, it is best to utilize operators familiar with the instrument, and comfortable with alignment. The operator conducting the method should align the optics and illumination for their use, with supervisory/trainer approval.
It is recommended to start with alignment of the microscope for a typical transmitted illumination observation using a known sample. Any specimen familiar to the operator will suffice; however, a common particle count reference standard suspension such as the USP PCRS is a recommended selection, since it is also utilized in method system suitability evaluation. A drop of the USP PCRS is placed between a glass microscope slide and cover slip and viewed microscopically.5 With appropriate interpupillary distance and a comfortable sitting position at the microscope, the operator examines the fields of suspended spheres. One should observe the small standard spheres crisply in a combined field (with ease) for both eyes. One attains crisp focus and ease of view after separate focal adjustment of each eyepiece focus for a single point on the specimen.
Rotate the graticule in the right microscope eyepiece so that the linear scale is located at the bottom of the field of view, bringing the graticule into sharp focus by adjusting the right eyepiece diopter ring while viewing an out-of-focus specimen. Focus the microscope on a specimen, looking through the right eyepiece only. Then, looking through the left eyepiece, adjust the left eyepiece diopter to bring the specimen into sharp focus.
When the operator is not comfortable using the microscope or does not attain an equivalent crisp focus for each eye in a merged field of view, the counting will become a difficult experience and fatigue and flawed size comparison will result.
Nothing is better for preparing the operator for counting particles than to examine a test membrane as a positive control. Seasoned microscopists may not require this step, but for new operators or individuals conducting many different types of methods in the modern laboratory, familiarization is a prudent exercise. A filter membrane of the type being used for the method, such as a 25-mm color-contrast, plain 0.45-µm nominal pore size, containing particles, is a good choice. This may be a sample from previous method that contains a variety of particle types, or one prepared for familiarization. This positive test control will contain natural particles (flakes, threads, equant particles, various colors/opacity, a range of sizes, etc.) to effectively refresh the operator's sensitivity and facilitate microscope and illumination alignment for optimal viewing.
One would examine the membrane preparation, locate a typical array of particles and first bring the illumination into good alignment:
-
Adjust the external, incident illumination at an oblique angle (10–20
 to the method) so that an even ellipse of reflected light is visible on the membrane and an even illumination evident through the eyepiece field of view (even across the full field). Shadows will be evident from larger particles, such as those with z axis > 5µm (z axis is the microscope optical axis).
to the method) so that an even ellipse of reflected light is visible on the membrane and an even illumination evident through the eyepiece field of view (even across the full field). Shadows will be evident from larger particles, such as those with z axis > 5µm (z axis is the microscope optical axis).
- Adjust the internal episcopic brightfield illuminator to yield an even illumination at a high setting on the transformer control, but more importantly, when dialing down the illumination one observes the evident shadow from the larger particles. In this manner, the high reflectivity of flat, glassy particles (find one) and the distinct shadows of more equant (x:y:z ~ 1:1:1) particles is evident.
using the circular diameter graticule
The USP graticule is specifically fabricated for each microscope. The relative error of the graticule used must be ±2% and is initially measured with an NIST-certified stage micrometer. To accomplish this, align the graticule micrometer scale with the stage micrometer so that they are parallel. (Compare the scales, using as large a number of graduations on each as possible.) Read the number of graticule scale divisions, GSD, compared to stage micrometer divisions, SMD. Calculate the relative error by the formula:
100[(GSD  SMD)/SMD]
SMD)/SMD]
A relative error of ±2% is acceptable and verifies good alignment, focus and proper magnification. Thereafter, a day-of-use verification by the microscope operator with the NIST stage micrometer or commercial stage micrometer is sufficient to demonstrate proper setup.
The basic technique of measurement applied with the use of the circular diameter graticule is to count all particles 10 µm and larger, further categorizing in  10 µm and
10 µm and  25 µm. The circular zone or graticule field of view is a useful zone for active sizing and counting. Particles are compared to the linear scale and/or circles to determine their size in equivalent circular diameter. This is conducted by transforming mentally the image of each particle into a circle and then comparing to the 10- and 25-µm graticule reference circles. The sizing process is carried out without superimposing the particle on the reference circles; particles are not moved from their locations within the graticule field of view (the large circle) for comparison to the reference circles. Compare the area of the particle being sized to that of the black or transparent circles. Use the area of the clear graticule reference circles to size white or transparent particles. Use the area of the black reference circles to size dark particles. The intent of comparing particles to an equivalent circular diameter is correlation to the LO particle sizing methodology, for which many manufacturers have extensive databases. In practice, particles with nearly circular areas will correlate well with the graticule circle diameters. For particles with one long axis, such as rods and needles, the conversion to circular area will produce more significant bias to smaller estimated sizes. It may be simpler, and most conservative, to count particles in longest chord. To use an extreme example, the total count of mono-dispersions of fine needle crystals would vary greatly dependant upon the size determination utilized.
25 µm. The circular zone or graticule field of view is a useful zone for active sizing and counting. Particles are compared to the linear scale and/or circles to determine their size in equivalent circular diameter. This is conducted by transforming mentally the image of each particle into a circle and then comparing to the 10- and 25-µm graticule reference circles. The sizing process is carried out without superimposing the particle on the reference circles; particles are not moved from their locations within the graticule field of view (the large circle) for comparison to the reference circles. Compare the area of the particle being sized to that of the black or transparent circles. Use the area of the clear graticule reference circles to size white or transparent particles. Use the area of the black reference circles to size dark particles. The intent of comparing particles to an equivalent circular diameter is correlation to the LO particle sizing methodology, for which many manufacturers have extensive databases. In practice, particles with nearly circular areas will correlate well with the graticule circle diameters. For particles with one long axis, such as rods and needles, the conversion to circular area will produce more significant bias to smaller estimated sizes. It may be simpler, and most conservative, to count particles in longest chord. To use an extreme example, the total count of mono-dispersions of fine needle crystals would vary greatly dependant upon the size determination utilized.
In order to properly focus the ocular lenses and attain balanced single-field view, each operator must bring the USP graticule lines into sharp focus by adjusting the eyepiece diopter ring (it helps to have an “infinite” view,or out-of-focus specimen). Next, focus the microscope on a specimen, through this same eyepiece, and then looking only through the other eyepiece, adjust its diopter ring to bring the specimen into sharp focus. The USP graticule and specimen particles are now in focus on a well-balanced illumination field.
Preparation of Filtration Apparatus and Test Preparations are covered by  788
788 . Further, prepare the test specimens in the following sequence. Outside of the unidirectional airflow cabinet to be used for the test, remove outer closures, sealing bands, and remove or tape over labels. Rinse the exteriors of the containers with filtered water as directed under Test Environment. Protect the containers from environmental contamination until analyzed.
. Further, prepare the test specimens in the following sequence. Outside of the unidirectional airflow cabinet to be used for the test, remove outer closures, sealing bands, and remove or tape over labels. Rinse the exteriors of the containers with filtered water as directed under Test Environment. Protect the containers from environmental contamination until analyzed.
Within the HEPA cabinet, open and withdraw, pour, or otherwise sample the contents of the containers under test in a manner least likely to generate particles that could enter the test specimen. Contents of containers with removable stoppers may be withdrawn directly by removing the closures. Sampling devices having a needle to penetrate the unit closure may also be employed. Products packaged in flexible plastic containers may be sampled by cutting the medication or administration port tube or a corner from the unit with a suitably cleaned razor blade or scissors.
For all products, regardless of volume, comprehensive experience regarding the integrity and consistency of the batch is gained throughout development, allowing the proper sampling plans to be applied in commercial production that ensure sample selection is representative of batch quality. All batches must have sampling plans that accommodate desired statistical measures of batch quality and facilitate process control.
product particle count determination
Depending upon the dosage form being tested, proceed as directed under the appropriate category below.
Liquid Preparations—
Thoroughly mix the units to be tested by inverting 20 times. Open the units in a manner consistent with the generation of the lowest possible numbers of background particles. For products less than 25 mL in volume, one may open them and drain to the filtration barrel individually, or combine the contents of 10 or more units in a cleaned container. [note—When pooling containers, these must be included in the blank determination step. ] Filter large-volume injection units individually. Small-volume injection units having a volume of 25 mL or more may be filtered individually.
Transfer to the filtration funnel the total volume of a solution pool or of a single unit, and apply vacuum. If the volume of solution to be filtered exceeds the volume of the filtration funnel, add, stepwise, a portion of the solution until the entire volume is filtered. It is prudent to maintain the liquid volume in the filtration funnel above one-half of the funnel volume between refills, especially if the partial count procedure is to be used (see Enumeration of Particles, Partial Count Procedure, below). [note—This is necessary in order to ensure even distribution of particles on the analytical membrane. ] After the last addition of solution, begin rinsing the walls of the funnel by directing a low-pressure stream of filtered water in a circular pattern along the walls of the funnel, and stop rinsing the funnel before the volume falls below about one-fourth of the fill level. Maintain the vacuum until all the liquid in the funnel is gone.
Remove the filtration funnel from the filtration base while maintaining vacuum, then turn the vacuum off, and remove the filter membrane with non-serrated forceps. Place the filter in the prepared holder and label with sample identification. Allow the filter to air-dry in the unidirectional airflow enclosure with the cover ajar.
Dry or Lyophilized Preparations—
Prepare the containers as directed under Test Preparation. Open each container, taking care not to contaminate the opening or cover. Constitute as directed by the labeling, according to the Test Preparation. Alternately, depending on the experiment, use:
- filtered water or an appropriate laboratory-filtered diluent if suitable.
Products Packaged with Dual Compartments Constructed to Hold the Drug Product and a Solvent in Separate Compartments—
Activate each unit as directed in the labeling, agitating the contents sufficiently to ensure thorough mixing of the separate components, and then proceed as directed for Liquid Preparations.
Pharmacy Bulk Packages or Multiple-Dose Containers—
For Products Labeled “Pharmacy Bulk Package—Not for Direct Infusion” or for multiple-dose containers, proceed as directed for Liquid Preparations, filtering the total unit volume.
Calculate the test result on a portion that is equal to the maximum dose given in the labeling. Consider this portion to be the equivalent of the contents of one full container. For example, if the total bulk package volume is 100 mL and the maximum dose listed is 10 mL, the microscope total unit volume count test result would be multiplied by 0.1 to obtain the test result for the 10-mL dose volume. [note—For calculation of the test result, consider this portion to be the equivalent of the contents of one full container. ]
Enumeration of Particles
The microscope test described in this section is flexible in that typical artifacts such as air and immiscible liquids do not interfere with the final count. The method has a broad size detection and counting range, if applying the partial count procedure. This method may be used where all particles on an analysis membrane surface are counted or where only those particles on some fractional area of a membrane surface are counted.
total count procedure
The microscope method can be tedious (boring), imprecise (poor agreement within and between labs) and particle sizing can be inaccurate for non-spherical or equant particle shapes. Operator fatigue is promoted by poor ergonomic fit (chair height), by poor or imbalanced ocular focus and by excessive eye movement. Restricting the eye movement to a field-defining graticule such as the USP counting graticule restricts eye movement to the central one-third field of view. This significantly limits eye movement and thus fatigue.
Sample size is an important consideration in counting precision. Care must be taken to sample many containers within a batch for good representation of the particle distribution. Accordingly, the portion of the individual package sampled is key. Particles may float or settle. Sampling only the first 25 mL of a LVP or sampling without adequate and recent mixing is a mistake, and will lead to serious under-counting. Sampling whole, well-mixed containers with the particles in suspension is the best approach.
Counting the isolated particles is an important parameter. Counting all of the particles retained on the membrane is certainly the best approach, and then the simple problem is determining the correct size for placement into the threshold bins, 10 µm and 25 µm. This will be increasingly important for methods utilizing additional bins for population determination, such as 5 µm, 50 µm,100 µm, etc. Note that the particle limits for  788
788 and
and  789
789 must be reported as all particles
must be reported as all particles  10 µm and all particles
10 µm and all particles  25 µm. If the lab method has been configured to count in several bins, such as
25 µm. If the lab method has been configured to count in several bins, such as  10–25 µm,
10–25 µm,  25–50 µm,
25–50 µm,  50 µm, etc., all bins
50 µm, etc., all bins  10 µm must be added to yield total
10 µm must be added to yield total  10 µm count; all bins
10 µm count; all bins  25 µm need to be added to yield total count
25 µm need to be added to yield total count  25 µm. Using a number of narrow size bins may be beneficial in product improvement efforts to separate particle groups.
25 µm. Using a number of narrow size bins may be beneficial in product improvement efforts to separate particle groups.
In performance of a total count, the graticule field of view (GFOV) is defined by the large circle of the graticule, and the vertical crosshair is used as a counting target. Scan the membrane in paths that cover the effective filtration area (EFA), adjoining but not overlapping previous scan paths. Repeat this procedure, tabulating particle counts minimally in the  10 µm–25 µm and
10 µm–25 µm and  25-µm thresholds, moving across the membrane until all particles on the membrane within the EFA are counted. Record the total number of particles that are
25-µm thresholds, moving across the membrane until all particles on the membrane within the EFA are counted. Record the total number of particles that are  10 µm–25 µm and the number that are
10 µm–25 µm and the number that are  25 µm or larger.
25 µm or larger.
For large-volume products, calculate the particle count, in particles per mL, for each unit tested by the formulas:
PS 10/V
10/V
PS 25/V
25/V
in which PS 10 is the total particle count obtained from all portions analyzed, PS
10 is the total particle count obtained from all portions analyzed, PS 25 is the total particle count obtained from all portions
25 is the total particle count obtained from all portions  25 µm analyzed, and V is the volume, in mL, of the solution tested.
25 µm analyzed, and V is the volume, in mL, of the solution tested.
For example, the analyst has counted the test samples in four bins: (a)  10–25 µm, (b)
10–25 µm, (b)  25 µm–50 µm, (c)
25 µm–50 µm, (c)  50 µm –100 µm, and (d)
50 µm –100 µm, and (d)  100 µm. They would then calculate as:
100 µm. They would then calculate as:
PS 10 = P
10 = P 10–25 µm + P
10–25 µm + P 25 µm –50 µm + P
25 µm –50 µm + P 50 µm –100 µm + P
50 µm –100 µm + P 100 µm
100 µm
For small-volume products, calculate the particle count, in particles per container, by the formulas:
PS 10/n
10/n
PS 25/n
25/n
in which PS 10 is the total particle count obtained from all portions analyzed, PS
10 is the total particle count obtained from all portions analyzed, PS 25 is the total particle count obtained from all portions
25 is the total particle count obtained from all portions  25 µm analyzed, and n is the number of units pooled (1 in the case of an individual unit).
25 µm analyzed, and n is the number of units pooled (1 in the case of an individual unit).
partial count procedure
When encountering a membrane full of particles, the task of counting all of them, properly, is daunting. Consider that an SVP with an at-limit content of small particles, sampled in a 10-vial pool would have 30,000 10-µm particles on the membrane. Partial or statistical counting of the membrane effective filtration area may be the only means to attain reasonable results. Partial counting should not be used to reduce count times, just as a means to estimate the total load on a high-count isolate. A field-defining device, such as grids on the membrane surface or an ocular graticule field of view have been used reliably. An ocular graticule provides a sharp boundary for area definition. Gridded membrane lines are rather broad and have ink-spatter that may be taken for particulate matter.
Which portions and how much of the EFA should be counted? In consideration of 25-mm membranes, the EFA is 16-mm diameter using typical commercial filtration funnels, and thus (pi × r2) = 201 mm2. Based upon earlier proposals from the HIMA committee and discussion by Draftz (see Reference 3), acceptable confidence intervals (Poisson distribution, 2 standard deviations) dictate that for samples with less than 1000 particles, the imprecision of statistical counting is objectionable. Full count is recommended for such samples. For samples with more than 1000 particles on the isolate membrane, using a 25-mm membrane, a reasonable estimate of particle population is attained using 20 GFOV. If a smaller confidence interval about the result is desired, a larger number of fields and particles may be counted.
For 47-mm membranes, the EFA is 37 mm. These larger diameter membranes may be selected for formulations needing more membrane surface area (having slow flow characteristics through 25-mm membranes) the EFA = (pi × r2) = (pi × 18.5 mm2) = 1075 mm2. Thus, for 47-mm membrane EFAs, many more GFOVs must be counted to attain similar confidence. Using 100 GFOVs for partial counting of 47-mm membranes provides similar statistical confidence to the 20 GFOV/25-mm approach. Accordingly, when a particle load of 1000 or less is present, a full count is recommended.
When a partial count of particles on a membrane is to be performed, the analyst must first ensure that an even distribution of particles is present on the membrane. This is assessed by rapid scanning at 50× to qualitatively scan for heterogeneity or clumps of particles. If heterogeneity is observed, one should perform a full count on the membrane. Next, count the 10-µm or larger particles in one GFOV at the edge of the filtration area as well as one in the center of the membrane. The number of  10-µm or larger particles in the GFOV with the highest total particle count must not be more than twice that of the GFOV with the lowest particle count. Fully count the membrane failing these criteria.
10-µm or larger particles in the GFOV with the highest total particle count must not be more than twice that of the GFOV with the lowest particle count. Fully count the membrane failing these criteria.
To perform a partial count of the particles on a membrane, Include all particles  10–25 µm and
10–25 µm and  25 µm within the GFOV and those that are in contact with the right side of the GFOV circle. Do not count particles outside of the GFOV. Ignore those that touch the left side of the GFOV circle. The dividing line between right and left sides of the GFOV circle is the vertical cross hair and is a useful counting line. [Note—Make the best possible judgment on particle size without changing the membrane position, microscope magnification or illumination. ]
25 µm within the GFOV and those that are in contact with the right side of the GFOV circle. Do not count particles outside of the GFOV. Ignore those that touch the left side of the GFOV circle. The dividing line between right and left sides of the GFOV circle is the vertical cross hair and is a useful counting line. [Note—Make the best possible judgment on particle size without changing the membrane position, microscope magnification or illumination. ]
Start at the center edge of the filtration area and begin counting adjacent GFOVs. When the other edge of the filtration area is reached, move one GFOV toward the top of the filter and continue counting GFOVs by moving in the opposite direction. Moving from one GFOV to the next can be accomplished by one of two methods. One method is to define a landmark (particle or surface irregularity in the filter) and move over one GFOV in relation to the landmark. A second method is to use the vernier on the microscope method to move 1 mm between GFOVs. To facilitate the latter, adjust the microscope x- and y-method positioning controls to a whole number at the starting position at the center right edge of the filtration area, then each GFOV will be one whole division of movement of the x-method positioning control. If the top of the filtration area is reached before the desired number of GFOVs is reached, begin again at the right center edge of the filtration area one GFOV lower than the first time. This time move downward on the membrane when the end of a row of GFOVs is reached. Continue as before until the number of GFOVs is complete.
For large-volume products, extrapolate the total count of particles per mL by the formulas:
PS10AT/APV
PS25AT/APV
in which PS10 is the total particle count obtained from all fields of view and all size thresholds; PS25 is the total particle count obtained from all fields of view and all size thresholds  25 µm; AT is the filtration area, in mm2, of the membrane (inner filtration barrel diameter); AP is the partial area counted, in mm2, based on the number of graticule fields counted (GFOV area × number of GFOV counted); and V is the volume, in mL, of solution filtered.
25 µm; AT is the filtration area, in mm2, of the membrane (inner filtration barrel diameter); AP is the partial area counted, in mm2, based on the number of graticule fields counted (GFOV area × number of GFOV counted); and V is the volume, in mL, of solution filtered.
For a solution pool (for small-volume product units containing less than 25 mL) or for a single unit of a small-volume product, extrapolate the total count of particles per unit by the formulas:
PS10AT/APn
PS25AT/APn
in which PS10 is the total particle count obtained from all fields of view and all size thresholds, PS25 is the total particle count obtained from all fields of view and all size thresholds  25 µm, and n is the number of units counted (1 in the case of an individual unit). For all types of product, if the tested material has been diluted to decrease viscosity, the dilution factor must be accounted for in the calculation of the final test result.
25 µm, and n is the number of units counted (1 in the case of an individual unit). For all types of product, if the tested material has been diluted to decrease viscosity, the dilution factor must be accounted for in the calculation of the final test result.
References
1. Groves MJ. The formulation of parenteral products. In: Parenteral Products, the preparation and quality control of products for injection. William Heinemann Medical Books, Ltd. London, 1973: 43–45.
2. Knapp, J.Z and H.K. Kushner (1980). Generalized Methodology for Evaluation of Parenteral Inspection Procedures, J. Parenteral Drug Association, 34:14.
3. Draftz RG. “Microscopical Counting, Sizing and Statistical Strategies for LVP Contaminants,” in Conference Proceedings, International Conference on Liquid Borne Particle Inspection and Metrology, May 11-13, 1987, Arlington VA. pp. 458–466.
1
Passed through a filter having a nominal pore size of 1.2 µm or finer.
2
ASTM standard F658-00a provides useful discussions pertaining to calibration procedures applying near-monosize latex spheres.
3
Soft particles and semi-solid substances may also be retained.
4
N.A. is numerical aperture, an indicator of optical light-gathering capability, and thus resolution. High N.A. correlates to high resolution.
5
The microscope objective requires a defined cover slip thickness, nominally 170µm, or No. 1 ½.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Desmond G. Hunt, Ph.D.
Senior Scientific Liaison 1-301-816-8341 |
(GCDF2010) General Chapters - Dosage Forms |
USP35–NF30 Page 945
Pharmacopeial Forum: Volume No. 35(6) Page 1533