INTRODUCTION
This chapter considers sensitization and hypersensitization in the context of medical devices and implants, and describes methodologies for testing such articles for their potential to cause sensitization.
There are four types of hypersensitization reactions according to the Gell and Coombs classification system. Type I reactions involve the fixation of IgE to mast cells that subsequently release pharmacologically active substances, such as histamine. Type II reactions are the result of IgG and/or IgM binding to target cells, followed by complement fixation and cell lysis. Type III reactions are caused by the presence of antigen-antibody complexes that cause physical damage such as kidney damage due to glomerular blockage. Type IV reactions are cell-mediated (involve the action of T cells and their interaction with the human lymphocyte antigens). Type IV reactions are also called delayed-type hypersensitivity reactions. Table 1 below summarizes the types of reactions, the mediators of the reactions, and examples of representative diseases.
Table 1. The Four Types of Hypersensitization Reactions*, Mediators, and Disease Examples
| Reaction Class |
Mediators | Disease Examples |
|---|---|---|
| Type I | IgE molecules bound to mast cells interact with antigen to release pharmacologically active substances | Hay fever, bronchial asthma, other atopic reactions |
| Type II | IgM and/or IgM molecules interact with target cells, fix complement, cell lysis | Various drug allergies, erythroblastosis fetalis, hemolytic anemia, thrombocytopenia |
| Type III | Antigen-antibody complexes, complement | Arthus reaction, serum sickness, allergic glomerulonephritis |
| Type IV | T lymphocytes, antigen, monocytes, macrophages | Contact dermatitis |
|
*
According to Gell and Coombs classification scheme
|
||
A multi-step process, delineated in chapter The Biocompatibility of Materials Used in Drug Containers, Medical Devices, and Implants  1031
1031 is followed in determining which, if any, toxicological tests need to be performed on a given article. In some cases, sufficient evidence to satisfy toxicology requirements may be available from previously marketed articles (See Figure 1 in chapter
is followed in determining which, if any, toxicological tests need to be performed on a given article. In some cases, sufficient evidence to satisfy toxicology requirements may be available from previously marketed articles (See Figure 1 in chapter  1031
1031 ). Important factors addressed in Figure 1 (chapter
). Important factors addressed in Figure 1 (chapter  1031
1031 ) include the type and extent of contact with the body, the chemical composition, the manufacturing process, the sterilization process, and, as mentioned above, similarity to previously marketed articles.
) include the type and extent of contact with the body, the chemical composition, the manufacturing process, the sterilization process, and, as mentioned above, similarity to previously marketed articles.
If further toxicological testing is necessary, the classification of medical devices provided in Table 2 from general information chapter  1031
1031 is important, because the degree and extent of toxicological testing that is required is strongly influenced by the nature and duration of the bodily contact with the article. The classification derived from Table 2 in chapter
is important, because the degree and extent of toxicological testing that is required is strongly influenced by the nature and duration of the bodily contact with the article. The classification derived from Table 2 in chapter  1031
1031 , coupled with the length of exposure to the article, is used in Tables 3–5 of chapter
, coupled with the length of exposure to the article, is used in Tables 3–5 of chapter  1031
1031 to determine which toxicological tests need to be performed. Table 2 below presents information extracted from Tables 3–5 of chapter
to determine which toxicological tests need to be performed. Table 2 below presents information extracted from Tables 3–5 of chapter  1031
1031 and indicates those circumstances for which sensitization testing should be considered.
and indicates those circumstances for which sensitization testing should be considered.
Table 2. Articles For Which Sensitization Testing Should Be Considered Based on Article Category and Length of Exposure
| Device Category | Body Contact | Contact Duration |
|---|---|---|
| Surface devices | Skin | Aa, Bb, Cc |
| Mucosal membrane | A, B, C | |
| Breached or compromised surfaces | A, B, C | |
| External communicating devices | Blood path, indirect | A, B, C |
| Tissue, bone, or dentin communicating | A, B, C | |
| Circulating blood | A, B, C | |
| Implant devices | Tissue or bone | A, B, C |
| Blood | A, B, C | |
|
a
A: limited (less than 24 hours)
b
B: prolonged (24 hours to 30 days)
c
C: permanent (more than 30 days)
|
||
There are nine test methodologies reviewed in this chapter. Table 3 lists the methods and the species with which they are performed.
Table 3. Test Methodologies That May Be Used in Sensitization Testing, and Species Required for Test
| Test | Species Used in Test |
|---|---|
| Magnusson & Kligman Maximization | Guinea pig |
| Standard Buehler | Guinea pig |
| Open Epicutaneous | Guinea pig |
| Freund's Complete Adjuvant | Guinea pig |
| Optimization | Guinea pig |
| Split Adjuvant | Guinea pig |
| Local Lymph Node Assay | Mouse |
| Mouse Ear Swelling | Mouse |
| Vitamin A Enhancement | Mouse |
Given the preponderance of testing performed with either the Magnusson & Kligman Guinea Pig Maximization Test (GPMT) or Buehler Tests (BT), those tests will be reviewed in detail in this chapter. A brief summary of the remaining tests is provided as alternatives to the more frequently used procedures.
Each test should be periodically validated in the performing laboratory using positive controls such as hexyl cinnamic aldehyde, mercaptobenzothiazole, or benzocaine (positive controls recommended by the Organization for Economic Cooperation and Development [OECD]).
MAGNUSSON & KLIGMAN GUINEA PIG MAXIMIZATION TEST (GPMT)
Animals
Either male and female albino guinea pigs or both may be used. All animals should be in good health and weigh between 300 g and 500 g at the start of the experiment. The females should not be pregnant, nor should they have borne young previously. Prior to use, it is essential to acclimatize the animals to the laboratory conditions for at least 5 days. All animals should be handled in accordance with the guidelines in the appropriate regulatory requirements established for the humane treatment of animals. At least 10 test animals and 5 control animals should be used. To obtain sufficient analytical power (i.e., to detect weak sensitizers) it may be necessary to use 20 test animals and 10 control animals. Additional animals may be required to establish the proper doses to administer (see Determination of Test Article Concentration).
Housing and Feeding
The animal room should be held at 20 ± 3 , at 30% to 70% relative humidity, with 12 hours of light and dark. Animals may be housed individually or in group housing. Standard laboratory diets may be used (those satisfactory for guinea pigs ensure an adequate amount of ascorbic acid). Drinking water should be available ad libitum.
, at 30% to 70% relative humidity, with 12 hours of light and dark. Animals may be housed individually or in group housing. Standard laboratory diets may be used (those satisfactory for guinea pigs ensure an adequate amount of ascorbic acid). Drinking water should be available ad libitum.
Animal Pretest Preparation
Animals should be randomized via a validated randomization method. For example, such methods may utilize random number tables or computer-generated random numbers. Sites on the animals intended for test article application (intrascapular region) should have the hair removed in a manner that does not abrade the skin. This may be accomplished via clipping, shaving, or with chemical depilatories. The chemical depilatory must not elicit irritation of its own. General observations of the animals prior to use in the test should be recorded, including any indication of ill health (do not use such animals in tests), and body weights.
Test Article Preparation1
The use of this test requires that the test article can be injected intradermally. When the test article is not suitable for direct administration, extracts should be prepared according to the procedure provided in general chapter Biological Reactivity Tests, In Vivo  88
88 .
.
Determination of Test Article Concentration
The purpose of this preliminary study is to determine the concentrations of Test Article Preparation to be used during the initial induction phase and the second challenge phase of a GPMT study. Two or three animals may be used for the concentration determination.
A range of concentrations of the test article, or extracts of the article, should be injected intradermally (0.1 mL per site), using the solvent that will be employed in the Test Procedure. The concentration that causes only mild to moderate irritation (no extensive skin destruction, with no evidence of overt systemic toxicity to the animals) should be used in the Intradermal Injection Induction Phase of the Test Procedure.
Using two or more animals, apply via occlusive dressings and patches, a range of concentrations of test article or extracts of the article. Remove the dressings/patches after 24 hours, and examine the sites for erythema. Choose the concentration that causes only slight erythema for the Topical Application Induction Phase of the Test Procedure. Use the highest concentration of test article or extract that does not cause erythema for the Challenge Phase of the Test Procedure. If the irritation threshold is not reached, then select the highest possible concentration for the Topical Application Induction Phase and Challenge Phase of the Test Procedure.
Test Procedure
intradermal injection induction phase
This phase requires three pairs of injections administered intradermally, with the test and control injection of each pair on opposite sides intrascapularly. Each injection should contain 0.1 mL, with injection pairs 1 and 2 administered nearer to the head, and injection pair 3 administered slightly farther towards the tail. The pairs are nominally within an area of 8 cm2. The pairs of injections consist of the following:
| Injection pair 1: | A 1:1 (v/v) mixture of Freund's Complete Adjuvant (FCA), an oil–water emulsion containing mycobacteria, and the appropriate solvent/vehicle (see Biological Reactivity Tests, In Vivo |
| Injection pair 2: | The Test Article Preparation in the concentration as specified in Determination of Test Article Concentration, using the appropriate solvent/vehicle. Control animals receive only the solvent/vehicle. |
| Injection pair 3: | The Test Article Preparation in the concentration as specified in Determination of Test Article Concentration in a 1:1 (v/v) mixture with FCA. Control animals receive an injection of a 1:1 (v/v) mixture of FCA and solvent/vehicle. |
topical application induction phase
Seven days (±1 day) after completion of the Intradermal Injection Induction Phase, administer the test sample by topical application to the intrascapular region of each animal. For both test and control animals, if the Test Article Preparation does not cause skin irritation, apply 10% sodium lauryl sulfate in petrolatum approximately 24 hours before the start of the Topical Application Induction Phase to induce a local irritation.
Test animals should have 2- × 4-cm pieces of filter paper or absorbent gauze fully loaded with the Test Article Preparation (prepared within 24 hours of use) using the concentration selected in Determination of Test Article Concentration applied to each injection site. The filter paper or absorbent gauze should be secured to the animals using occlusive dressings. Control animals receive the same treatment, except that the appropriate solvent/vehicle is used instead of the test article.
Remove the dressings and patches approximately 48 hours after application.
challenge phase
This phase should occur 14 ± 1 days after the Topical Application Induction Phase. Hair should be removed from the test application sites. Filter paper patches or chambers are soaked with a freshly prepared Test Article Preparation in the concentration specified in Determination of Test Article Concentration. This is done for all test and control animals. The patches or chambers are secured with an occlusive dressing and removed after 24 ± 2 hours.
Observations
At approximately 24, 48, and 72 hours after removal of the challenge patches, the application sites should be examined for signs of reactions. Of particular importance are instances where the reaction of the test animals exceeds that of the control animals. All signs of reactivity should be recorded, with particular attention paid to signs of erythema and edema. A true edematous reaction will blanch under gentle pressure. The longer the period of blanching, the greater the severity of edema.
Interpretation
There is more than one way of evaluating and grading the results from GPMT. Tables 4, 5, and 6 list details for three such grading systems. Grades of 1 or higher in the test animals, with grades of less than 1 in control animals, are indicative of sensitization. If control animals display grade 1 reactivity, and if the test animals display reactivity above the greatest reactivity seen in the control animals, sensitization due to the test article is again suspected. The percentages in Table 4 need to be revised if there are only 10 test animals (i.e., the categories would be 0, <10%, 10%–30%, 31%–60%, 61%–80%, and 81%–100%.) If there are 20 test animals, then multiples of 5% are appropriate.
Table 4. Classification Based on Percent of
Responsive Test Animals
Responsive Test Animals
| % of Positives in Test Group |
Assigned Grade Class | |
|---|---|---|
| 0 | — | Nonsensitizer |
| <8 | 1 | Weak |
| 8–28 | 2 | Mild |
| 29–64 | 3 | Moderate |
| 65–80 | 4 | Strong |
| 81–100 | 5 | Extreme |
Table 5. Classification Based on Erythema and Edema Formation
| Erythema and Eschar | Grade |
| No erythema | 0 |
| Slight or equivocal erythema | <1 |
| Well-defined erythema | 2 |
| Moderate erythema | 3 |
| Severe erythema to slight eschar formation | 4 |
| Edema | |
| No edema | 0 |
| Slight or equivocal edema | <1 |
| Well-defined edema | 2 |
| Moderate edema | 3 |
| Severe edema | 4 |
Table 6. Classification Based on Erythema
Formation Alone
Formation Alone
| Erythema formation | Grade |
|---|---|
| No erythema | 0 |
| Discrete or patchy erythema | 1 |
| Moderate and confluent erythema | 2 |
| Intense erythema and swelling | 3 |
The results should be submitted for statistical analysis (e.g., chi-square contingency table) to determine if the differences in scores between treated and control animals are significant. The response of the test group versus the control group should be compared statistically. (The Mann-Whitney U test can be used for the comparison.)
Rechallenge
The extent of any response in the negative control group, under experimental conditions, shows the irritation potential of the Test Article Preparation. In this case, test and control animals should be rechallenged 1 week later on the untreated side of the animal, with a reduced concentration of the Test Article Preparation. A sensitized guinea pig will react to some degree to both challenges. A weak reaction occurring at a single time point in only one challenge should cast strong doubt as to whether that guinea pig is truly sensitized.2
STANDARD BUEHLER TESTS (SBT)
Animals
See Animals in the Magnusson & Kligman Guinea Pig Maximization Test (GPMT).
Housing and Feeding
See Housing and Feeding in the Magnusson & Kligman Guinea Pig Maximization Test (GPMT).
Animal Pretest Preparation
See Animal Pretest Preparation under Magnusson & Kligman Guinea Pig Maximization Test (GPMT). The fur of the guinea pig may be removed from one flank by clipping.
Test Article Preparation
See Test Article Preparation in the Magnusson & Kligman Guinea Pig Maximization Test (GPMT).
Determination of Test Article Concentration
The purpose of this preliminary study is to determine the concentrations of Test Article Preparation to be used during the initial induction phase and the second challenge phase of an SBT study. Two or three animals may be used for the concentration determination.
A range of concentrations of the test article, or extracts of the article, should be applied using patches (for example, four 4 cm2 absorbent pads) or chambers. The patches should be held in place using tape (if necessary) and occlusive dressings. The patches should be removed after approximately 6 hours, and any residues of the test chemical are removed from the test site. Observations are made at that time, and at 24 and 48 hours.
The concentration that causes only mild to moderate irritation (slight erythema, with no evidence of overt toxicity to the animals) and can be applied repeatedly to the same site should be used in the Induction Phase of the Test Procedure. Use the highest concentration of test article or extract that does not cause erythema for the Challenge Phase of the Test Procedure.
Test Procedure
induction phase
Apply 0.4 mL of the Test Article Preparation in an appropriate solvent/vehicle at the dose identified in Determination of Test Article Concentration. Use patches similar to those used in Determination of Test Article Concentration. The patches should be applied to one flank (hair clipped off) and held in place occlusively for 6 hours. The animals may need to be restrained to ensure occlusion. Patches and any visible residues should be removed after 6 hours. Control animals also receive patches, but these contain only the appropriate solvent/vehicle. This process should be repeated three times a week for both test and control animals on the same site for three consecutive weeks (weekly intervals are used in the modified Buehler Test).
challenge phase
This phase should be carried out 14 days after the last application of the Induction Phase. Clip the hair off the previously untested flank of each animal 24 hours before the challenge application. As in the Induction Phase, apply patches containing the test article (concentration specified in Determination of Test Article Concentration) or solvent/vehicle alone to the untested areas of the test and control animals. To obtain well-defined edges at the application sites, commercial chambers with a lipped edge are preferred. Secure the patches with occlusive dressings, and keep them in place for 6 hours. Remove all patches after 6 hours.
Observations
At 22 ± 2 hours after removal of the patches, the application sites should have the animal's fur removed via clipping or depilation. After approximately 2 more hours, grade the sites (Tables 4, 5 or 6 may be employed). All signs of reactivity should be recorded, with particular attention paid to signs of erythema and edema. Repeat the grading once again after 24 to 48 hours more have elapsed. The response of the test group versus the control group can be compared statistically. (The Mann-Whitney U test can be used for the comparison.)
Interpretation
The results should be submitted for a statistical analysis (e.g., chi-square contingency table) to determine if the differences in scores between treated and control animals are significant.
See Interpretation in the Magnusson & Kligman Guinea Pig Maximization Test (GPMT).
Rechallenge
See Rechallenge in the Magnusson & Kligman Guinea Pig Maximization Test (GPMT).
OTHER SENSITIZATION TEST PROCEDURES
The Magnusson & Kligman Guinea Pig Maximization Test and the Standard Buehler Tests are the most frequently performed sensitization tests. However, there are a number of other methods that may be useful in the assessment of the potential for sensitization. Some may be applicable to both solid test articles and extracts, some only to extracts.
Where the use of guinea pigs is called for in the following tests, the animals and their housing should meet the requirements as specified for Animals in the Magnusson & Kligman Guinea Pig Maximization Test. The fur of the guinea pig should be removed from test sites as indicated for Animal Pretest Preparation in the Magnusson & Kligman Guinea Pig Maximization Test.
Draize Test
This was the first predictive test accepted by the regulatory agencies, and is still in use. The test uses guinea pigs and the test article is administered via intradermal injections.
test article preparation
This test requires that the test article be in the form of a solution that may be directly applied to the animal's skin. Therefore, extracts of the material would need to be made. See Biological Reactivity Tests, In Vivo  88
88 for information on the preparation procedure.
for information on the preparation procedure.
induction phase
One flank of each of 20 guinea pigs is shaved, then 0.05 mL of a 0.1% solution of test article is injected into the anterior flank. The next day, and then every other day thereafter up to day 20, 0.1 mL of the test article is injected into a new site on the same flank.
challenge phase
This phase begins 2 weeks after the final injection of the Induction Phase. The untreated flank is shaved, then 0.05 mL of test article is injected into each of the 20 guinea pigs. Twenty previously untreated animals serve as the controls, and receive injections of the test article as well.
observations
The test sites of all control and test animals are evaluated for erythema at 24 and 48 hours after the challenge injections. The degree of reaction in test animals is compared to the reaction in control animals. A larger and/or more intense response by the test animals versus the control animals is indicative of sensitization.
Open Epicutaneous Test
This test uses guinea pigs. The goal is to determine the dose required to induce sensitization by simulating human usage via topical application of the test article.
test material preparation
This test requires that the test article be in the form of a solution that may be directly applied to the animal's skin. Therefore, extracts of the material need to be made. See Biological Reactivity Tests, In Vivo  88
88 for information on the preparation procedure.
for information on the preparation procedure.
preliminary testing
A series of concentrations of test article is applied to 2 cm2 areas of skin on the anterior flank of 6 to 8 guinea pigs (0.025 mL per application). The test sites should be examined for erythema 24 hours after test article administration. The highest concentration that does not cause irritation (maximum nonirritant concentration) and the lowest concentration causing erythema in approximately 25% of the animals (minimum irritant concentration) are determined.
induction phase
The test article (or control vehicle) is applied to 8 cm2 areas of the flank skin of 6 to 8 guinea pigs daily for 3 weeks, or five times a week for 4 weeks. The amount per application is 0.01 mL. A set of increasing concentrations is again employed, ranging from the minimum irritant concentration using a stepwise progression. The test article should be applied to the same sites each time, unless irritation develops, in which case a new site on the same flank should be used. Control animals receive the same series of treatments using the vehicle instead of the test article.
challenge phase
Each animal is challenged on the untreated flank 24 to 72 hours after the last Induction Phase treatment using 0.025 mL applied to 2 cm2 areas. A set of increasing concentrations is used, from minimum irritant concentration to the maximum nonirritant concentration, and five lower concentrations are also used.
observations
The test sites are evaluated at 24, 48, and 72 hours post-treatment. The maximum concentration that does not cause irritation in the control group is determined. Animals from the test groups that develop inflammatory responses at concentrations lower than the maximum nonirritating concentration in the controls should be considered to be sensitized.
Freund's Complete Adjuvant Test
This test is based upon the use of intradermal injections using the test article in a mixture of Freund's complete adjuvant and distilled water (50:50).
test material preparation
Because this test uses intradermal injections, extracts of the test material need to be made in order to use this procedure. See Biological Reactivity Tests, In Vivo  88
88 for information on the extraction procedure.
for information on the extraction procedure.
preliminary testing
The minimum irritating and the maximum nonirritating concentrations are determined in the same manner as for Preliminary Testing in the Open Epicutaneous Test.
induction phase
The test area consists of six 2 cm2 areas across the shoulders of the guinea pigs. Two groups of 10 to 20 guinea pigs each should be used. The test group animals are injected intradermally with 0.1 mL of a 5% solution of the test article extract in FCA/water. Control animals receive injections with FCA/water without the test article. These injections are repeated every 4 days until a total of three injections have been given.
challenge phase
This phase should begin 2 weeks after the last injection of the Induction Phase. Topical applications of 0.025 mL of test article at the minimum irritating and the maximum nonirritating concentrations, plus two lower concentrations, are administered to 2 cm2 areas of the shaved flank. The test sites should remain uncovered.
observations
The test sites are examined for the presence of erythema 24, 48 and 72 hours after the topical applications. The minimum nonirritating concentration in the control animals should be determined. Those test animals that display erythema at concentrations lower than the minimum nonirritating concentration in the control animals should be considered to be sensitized.
Optimization Test
This test has some similarities to the older Draize Test. Unlike the Draize Test, however, this test uses both intradermal and topical treatments, and includes adjuvant for some induction injections.
test material preparation
As with other test procedures that incorporate intradermal injections, the test article needs to be in a form suitable for injection. See Biological Reactivity Tests, In Vivo  88
88 for information on the extraction procedure.
for information on the extraction procedure.
induction phase
Twenty test and 20 control guinea pigs are used. A total of 10 intradermal injections should be given to each animal. Test animals receive 0.1 mL of a mixture of 0.1% test article and 0.9% saline (50:50) on day 1, with one injection into a shaved flank, and another into a portion of shaved dorsal skin. Two and 4 days later, one intradermal injection of the test article in saline is given to eight new dorsal sites. Every other day during weeks 2 and 3, the test article is injected intradermally into 10 sites over the shoulders in a 50:50 mixture of saline and FCA. The same sequence of injections is given to the 20 control animals, except that no test article is included with the saline or saline/FCA injections.
challenge phase
Thirty-five days after the first injection, the animals are challenged topically with 0.1 mL of the 0.1% solution of test article in saline (for test animals). The control animals receive saline injections only. At 45 days after the first injection, a second topical challenge is given. A nonirritating concentration of test article (0.05 mL) is applied topically to a 1 cm2 area of untreated skin. This site should then be covered with a 2 cm2 piece of filter paper, after which an occlusive dressing should be applied. The patch should be removed after 24 hours.
observations
Twenty-four hours after each injection during week 1, the thickness of a fold of skin over the injection sites for each animal should be measured using a caliper (mm), and the two largest cross-diameters of each erythematous reaction should be recorded (mm). The reaction volumes are calculated by multiplying the fold thickness by the products of the two cross-diameters (expressed as µL). The mean reaction (+1 SD) volume during week 1 should be calculated for each animal.
Challenge reaction volumes are calculated for each animal following the injections at day 35. If an animal develops a challenge reaction volume greater than its mean reaction volume + 1 SD, it should be considered sensitized.
Following the patch testing challenge, the test sites are evaluated for erythema and edema. Evaluations should be made using Table 5.
The number of positive animals should be compared statistically with the pseudopositive control animals. This should be done for both intradermal injection results and patch testing results. The Fisher exact test may be used.
The results from the intradermal injections and the patch testing, following separate statistical analysis, may be combined and evaluated using Table 7 in order to classify a test article as a strong, moderate, or weak sensitizer; or not a sensitizer.
Table 7. Classification Scheme for Test Articles Based on the Optimization Test
| Intradermal % of Positive Animals |
Patch Test % of Positive Animals |
Classification |
|---|---|---|
| S*, > 75 | and/or S, > 50 | Strong sensitizer |
| S, 50–75 | and/or S, 30–50 | Moderate sensitizer |
| S, 30–50 | N.S.*, 0–30 | Weak sensitizer |
| N.S., 0–30 | N.S., 0 | Not a sensitizer |
|
*
S = significant; N.S. = not significant
|
||
Split Adjuvant Test
This test makes use of both FCA and skin damage. The test article is applied topically.
test material preparation
Because this test employs topical test article applications, the article can be either in solid or liquid form. If extracts are to be made, see chapter Biological Reactivity Tests, In Vivo  88
88 for extraction procedures.
for extraction procedures.
induction phase
Ten to 20 guinea pigs are used for both test and control groups. An area of back skin immediately behind the scapulas should be shaved to the extent that the skin becomes glistening. The shaved areas should then be treated with dry ice for 5 to 10 seconds. A dressing made of loose mesh gauze with stretch adhesive and a 2- × 2-cm opening should be placed over the treated area, then secured with adhesive tape. The test article (0.2 mL of viscous materials, 0.1 mL of liquids, or solid material) is placed within the opening in the dressing on top of the treated skin. Two layers of #2 filter paper should be placed over the test article, then backed by occlusive tape. Then the filter paper/occlusive backed material should be secured to the surrounding dressing with adhesive tape. After 2 days have passed, the filter paper should be lifted from the test sites, and the test article reapplied on the same site. The filter paper and backing should be secured once again. After 2 more days, the filter paper should be lifted and two injections of 0.075 mL of FCA should be administered into the edges of the test site. Then the test material is once again applied, and the filter paper/backing resecured. The test article should be reapplied once more on day 7 and the filter paper/backing resealed. On day 9, the filter paper and all associated dressing material should be removed.
challenge phase
On day 22 following the induction treatment, 0.5 mL of test material (or the solid article) should be applied to a 2-× 2-cm area of shaved midback. The test sites should be covered by filter paper and backed by adhesive tape. This should be held in place with an elastic bandage secured with adhesive tape. Control animals receive the same challenge phase treatment. The preparation should be removed after 24 hours.
observations
Twenty-four, 48, and 72 hours after the removal of the challenge phase preparation, the test sites should be evaluated for erythema and edema. The grading scheme of Table 5 could be employed.
Mouse Ear Swelling Test
There are a number of potential advantages in using mice versus guinea pigs for sensitization methods. The classic guinea pig tests tend to be costly and require a long time to complete. Moreover, with the dependence upon relatively subjective scoring based on edema and erythema, methodological robustness, and ruggedness may be questionable. This test uses mice and employs both topical exposures and injections.
animals
Female, 6- to 8-week old CF-1, Balb/c, or Swiss mice should be used. They may be group housed in direct bedding cages. Acclimatization should be for at least 5 to 7 days. Food (appropriate mouse feed) and water should be available ad libitum. No animals with damaged pinnae should be used in the study. The thickness of both ears of each animal should be measured and recorded at this time.
test material preparation
As with other test procedures that incorporate intradermal injections, the test article needs to be in a form suitable for injection. See Biological Reactivity Tests, In Vivo  88
88 for information on the extraction procedure.
for information on the extraction procedure.
preliminary testing
The minimally irritating and maximally nonirritating concentrations of test article for this procedure should be determined. This is done by using four groups of two mice and examining the effects of at least four concentrations of test article.
induction phase
The abdomens of the animals should be shaved, then tape-stripped using a surgical adhesive tape until the test area is glistening. A single injection of 0.05 mL of FCA is subdivided into two injection sites administered intradermally within the shaved/stripped area, but along the borders. After the adjuvant injections, 100 µL of test article (using the minimally irritating concentration) or vehicle (controls) is applied to the center of the shaved test areas. After the test areas dry, the mice should be returned to their cages. The tape stripping and application of test article (but not FCA) is repeated each day for the next 3 days.
challenge phase
This phase should occur 7 days after the final topical induction application. The test article (highest nonirritating concentration) should be applied topically (20 µL) to one ear, while the opposite ear receives 10 µL of vehicle alone. This should be done for both test and control animals.
observations
The thickness of both ears of each animal should be recorded after 24 and 48 hours postchallenge. The measurements should be made with a caliper (a spring-loaded caliper is preferable). A sensitized animal is one in which the test article-treated ear is at least 20% thicker than its opposite ear. For the test to be valid, the test article-treated ears of control animals should not be more than 10% thicker than the opposite ears. If the control animal ears do not meet the requirements, the test should be repeated using lower concentrations.
Local Lymph Node Test
This test is based on the observation that exposure of the mice to sensitizers can cause hyperplasia of T cells within the auricular lymph nodes of mice. The method combines both in vivo and in vitro phases, and requires the use of radioisotopes. An unusual aspect of this test is that no challenge phase is required.
animals
Four groups of four mice at least, male or female CBA/ca mice (only one sex in a given test) between the ages of 8 to 12 weeks should be used.
test material preparation
Although in theory one could apply a solid test article to the dorsal surface of the ear of a mouse, in practice an extract of such an article should be used. See Biological Reactivity Tests, In Vivo  88
88 for information on the extraction procedure.
for information on the extraction procedure.
preliminary testing
A nontoxic concentration of test article should be used. If not already established, a preliminary test for overt toxicity may be required to establish a suitable dose.
induction phase
Twenty-five µL of the appropriate test article concentration, or vehicle (controls), should be applied to the dorsal surface of each pinna for 3 consecutive days. Five days after the first treatment, the animals should be injected, via the tail vein, with 2.5 mL of phosphate buffered saline containing 20 µCi of 3H-methyl thymidine. Five hours after the isotopic injection, the animals should be euthanized. The draining auricular lymph nodes should be removed from each animal of each test and control group. The nodes from all animals within a given group should be combined, such that a single cell suspension can be made from each group of animals. The cell suspension can be made by passing the nodes through a 200-mesh stainless steel gauze using a syringe plunger. The cells should then be centrifuged at 190 × g for 10 minutes, resuspended in 3 mL of 5% trichloroacetic acid (TCA), and held overnight at 4 .
.
The resulting precipitate should be recovered by centrifugation, and the pelleted precipitate should be resuspended in 1 mL of 5% TCA. The suspension should then be placed in scintillation vials with 10 mL of scintillation fluid, and the disintegrations/minute (dpm) counted with a 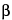 -counter.
-counter.
observations
The ratio of dpm for each test group should be compared to the dpm for the control group. If the ratio equals or exceeds 3 for any test group, the concentration of test article used with that group may be considered to be sensitizing.
Vitamin A Enhancement Test
This test is similar to the Mouse Ear Swelling Test in that test articles are applied topically to the abdomen, with a challenge application to the ears, followed by measurements of ear thickness. A principal difference is the use of mouse feed supplemented with vitamin A acetate. The purpose of the supplementation is to increase the reactivity of the immune system, thereby increasing the potential sensitization reaction.
animals
Male, 3- to 4-week old Balb/c mice should be maintained on a diet supplemented with vitamin A acetate. The diet may be prepared by mixing each kg of feed with 0.477 g of gelatinized vitamin A acetate. The feed mixture should be used within 3 weeks of preparation. Mice intended for use in sensitization studies should have been on the supplemented diet for at least 4 weeks. The mice at the time of the sensitization study should therefore be between 7 and 10 weeks old. The thickness of both ears of each animal should be measured and recorded at this time.
test material preparation
Although, in theory, one could apply a solid test article to the dorsal surface of the ear of a mouse, in practice an extract of such an article should be used. See Biological Reactivity Tests, In Vivo  88
88 for information on the extraction.
for information on the extraction.
preliminary testing
The maximally nonirritating dose and minimally irritating concentrations should be determined using separate groups of animals. This could be done as described for Preliminary Testing in the Mouse Ear Swelling Test.
induction phase
The fur of the abdomen and thorax of 10 mice per group should be shaved. Then 100 µL of test article (at the minimally irritating concentration) should be applied to the test areas on days 0, 2, 4, 7, and 11. Control animals receive 100 µL of vehicle alone on the same schedule.
challenge phase
This phase should occur 4 days after the final application of the Induction Phase. Twenty-five µL of test article (at the maximally nonirritating concentration) should be applied to each ear of each animal in the test and control groups.
observations
Ear thickness for both ears of each animal should be recorded after 24 and 48 hours postchallenge. The measurements should be made with a caliper (a spring-loaded caliper is preferable). The percent increase in ear thickness should be calculated for each ear by subtracting the pretreatment measurement from the post-treatment measurement, dividing the result by the pretreatment measurement, then multiplying by 100. The response of the test group versus the control group should be compared statistically. (The Mann-Whitney U test could be used for the comparison.)
The results of individual animals should also be calculated. If an increase in ear thickness for an animal from the test group is at least 50% greater than the largest increase of a control animal, that is indicative of sensitization. As an overall evaluation, should the results of the study provide a significant result of the statistical test at p < 0.01 for the control versus test group comparisons, or if at least two test animals have ear thickness increases in excess of 50% of the maximum control thickness changes and the group comparison showed a p < 0.05, sensitization is indicated for the test article.
1
For further information on sample preparation, see ANSI/AAMI/ISO/CEN Standard 10993–12—1996: Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials
2
Basketter D.A. Guinea pig predictive tests for contact hypersensitivity. In Immunotoxicology and Immunopharmacology, 2nd ed.; Dean, J.H, Luster, M.I., Munson, A.E., Kimber, I., Eds; Raven Press, Ltd: New York, 1994; pp 693–702.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(TOX2010) Toxicology |
USP35–NF30 Page 833
Pharmacopeial Forum: Volume No. 30(1) Page 289