Mission and Preface
USP 32–NF 27
USP 32–NF 27
This section provides background information on the United States Pharmacopeial Convention (USP), as well as general information about the 32nd revision of the United States Pharmacopeia (USP 32) and the 27th edition of the National Formulary (NF 27). Additional official information about the specific uses of these texts is provided in the General Notices and Requirements section, which has been significantly revised (see www.usp.org/GeneralNotices for a summary of revisions).
MISSION STATEMENT
USP–NF is published in continuing pursuit of the mission of USP: To improve the health of people around the world through public standards and related programs that help ensure the quality and safety of medicines and foods.
HISTORY
On January 1, 1820, 11 physicians met in the Senate Chamber of the U.S. Capitol building to establish a pharmacopeia for the United States. These practitioners sought to create a compendium of the best therapeutic products, give them useful names, and provide recipes for their preparation. Nearly a year later, on December 15, 1820, the first edition of The Pharmacopoeia of the United States was published. Over time, the nature of the United States Pharmacopeia (USP) changed from being a compendium of recipes to a compendium of documentary standards that increasingly are allied with reference materials, which together establish the identity of an article through tests for strength, quality, and purity. The publishing schedule of the USP also changed over time. From 1820 to 1942, the USP was published at 10-year intervals; from 1942 to 2000, at 5-year intervals; and beginning in 2002, annually.
In 1888, the American Pharmaceutical Association published the first national formulary under the title The National Formulary of Unoficinal [sic] Preparations (NF). Both the USP and the NF were recognized in the Federal Food and Drugs Act of 1906 and again in the Federal Food, Drug, and Cosmetic Act 1938. In 1975, USP acquired the National Formulary (NF), which now contains excipients standards with references to allied reference materials. Today, USP continues to develop USP and NF through the work of the Council of Experts into compendia that provide standards for articles based on advances in analytical and metrological science. As these and allied sciences evolve, so do USP and NF.
USP 32–NF 27
USP 32–NF 27 text is official May 1, 2009, unless otherwise noted. USP–NF contains official substance and preparation (product) monographs. The terms official substance and official preparation are defined in the General Notices of this Pharmacopeia. With few exceptions, all articles for which monographs are provided in USP 32–NF 27 are legally marketed in the United States or are contained in legally marketed articles.
A USP–NF monograph for an official substance or preparation includes the article's definition; packaging, storage, and other requirements; and a specification. The specification consists of a series of universal (description, identification, impurities, assay) and specific tests, one or more analytical procedures for each test, and acceptance criteria. Ingredients are defined as either drug substances or excipients. An excipient is any component, other than the active substance(s), intentionally added to the formulation of a dosage form. Excipients are not necessarily inert. Drug substances and excipients may be synthetic, semisynthetic, drawn from nature (natural source), or manufactured using recombinant technology. Larger molecules and mixtures requiring a potency test are usually referred to as biologicals or biotechnological articles.
USP 32–NF 27 contains approximately 4,303 monographs and more than 220 General Tests and Assays (General Chapters numbered 1,000 and below) and USP General Information Chapters (numbered above 1,000). General Chapters provide frequently cited procedures, sometimes with acceptance criteria, in order to compile into one location repetitive information that appears in many monographs. New and revised monographs and General Chapters and obsolete matter deleted from this edition are indicated in the Admissions section.
USP 32–NF 27 Organization—
USP 32–NF 27 is printed as a three-volume set. Volume 1 includes front matter (Mission and Preface, People, Governance pages and websites, Admissions/Annotations, and Commentary). It also includes USP General Notices, General Chapters, Dietary Supplement chapters, Reagents, Reference Tables, Dietary Supplement monographs, NF Admissions, Excipients, and NF monographs. Volume 2 includes USP monographs A–L, and Volume 3 includes USP monographs M–Z. Volumes 2 and 3 also include the USP General Notices and the Guide to General Chapters, and all three volumes include the full index. General Chapters specific to dietary supplements are included in numerical order with the rest of the General Chapters in USP. Excipient monographs usually are presented in NF but also may appear in USP with suitable cross-referencing when they are also drug substances. The Excipients section (Volume 1) presents a tabulation of excipients by functional category.
USP 32–NF 27 Spanish Edition—
In 2006, USP began providing an official Spanish edition of USP–NF. Maintenance of this edition follows the same revision approaches as the English edition.
Revisions—
USP–NF is continuously revised. Revisions are presented annually, in twice-yearly Supplements, in Interim Revision Announcements (IRAs), and in Revision Bulletins (on the USP website).
Supplements—
The First Supplement to USP 32–NF 27 will be published in February 2009 and will become official in August 2009. The Second Supplement will be published in June 2009 and will become official in December 2009. Users of USP print products must retain Supplements and subscriptions to Pharmacopeial Forum (PF) in order to have up-to-date information. The USP–NF online version is updated with each Supplement or annual revision. Each time a new edition or Supplement is released during the subscription period, a new CD-ROM will be issued. The Index in each Supplement is cumulative and includes citations to the annual revision and, for the Second Supplement, citations to the First Supplement. The contents of the two Supplements are integrated into the annual edition of the following year, along with new official revisions that have been adopted since the Second Supplement to the previous compendia.
Interim Revision Announcements (IRAs)—
IRAs contain revisions that become official in the interval between publication of the annual revision and Supplements, and thus provide an expedited mechanism for making revisions official. They appear in USP's bimonthly journal, Pharmacopeial Forum (PF), with the official date noted in the publication. They are subsequently incorporated into the next published Supplement or annual revision, although their official dates may precede the official date of that publication.
Revision Bulletins—
If the circumstances require immediate publication of official text, a proposal or postponement may be published through a Revision Bulletin. Revision Bulletins are posted on the USP website and published in the next USP-NF or Supplement, as applicable. Revision Bulletin official dates are specified in the individual Revision Bulletin.
Pharmacopeial Forum (PF)—
Each PF contains several sections. The Policies and Announcements section provides information about publication and comment deadlines, USP news, and summaries of issues discussed by the Council of Experts and its Expert Committees. Proposals for revision are presented as In-Process Revisions and represent draft revisions that are expected to advance to official status pending final review and approval by the relevant Expert Committee.
PF also includes Pending and Canceled Proposals and a Harmonization section. The Stimuli to the Revision Process section presents reports or statements of authoritative bodies, scientific articles relevant to compendial issues, general commentaries by interested parties, and summaries of comments received in response to policy initiatives. PF concludes with sections containing Nomenclature, Index, and Chromatographic Reagents used in USP–NF and PF. Each issue of PF also provides a cumulative index for the given calendar year.
Symbols Indicating Change to Official Text—
Symbols identify the beginning and end of each revision. The following table summarizes the types of symbols and the associated subscripts used in USP publications:
Interim revisions are shown with new text (if any) enclosed in circles,  new text
new text 1. New text revised in Revision Bulletins is enclosed in circles,
1. New text revised in Revision Bulletins is enclosed in circles,  new text(RB dd-mm-yyy). Text enclosed in squares,
new text(RB dd-mm-yyy). Text enclosed in squares,  new text
new text 1S (USP31), has already been adopted in a Supplement. Text that has been adopted in the USP–NF is enclosed in triangles,
1S (USP31), has already been adopted in a Supplement. Text that has been adopted in the USP–NF is enclosed in triangles,  new text
new text USP32. Where the symbols appear together with no enclosed text, such as
USP32. Where the symbols appear together with no enclosed text, such as 
 1 or
1 or 
 1S (USP31), it means that text has been deleted and no new text has been proposed to replace it.
1S (USP31), it means that text has been deleted and no new text has been proposed to replace it.
| Revision Type | Symbol | Subscript |
| Interim Revision Announcement | 1–6 | |
| Revision Bulletin | (RB 1-Jan-2009) | |
| Text deletion | ||
| Adopted in Supplement | 1 or 2S (USP annual edition) | |
| Adopted in USP–NF | USP annual edition |
In all revisions, the closing symbol is accompanied by a subscript number or date that indicates the Interim Revision Announcement (IRA), Revision Bulletin, or Supplement in which the revision first appeared. An example of a revision that was officially adopted in the Second Interim Revision Announcement would be  2; an example of a revision that was officially adopted in the Revision Bulletin on June 18, 2008, would be (RB 18-Jun-2008). An example of revision that was officially adopted in the Second Supplement to USP 31 would be
2; an example of a revision that was officially adopted in the Revision Bulletin on June 18, 2008, would be (RB 18-Jun-2008). An example of revision that was officially adopted in the Second Supplement to USP 31 would be  2S (USP31). Last, an example of a revision that was officially adopted in USP 32–NF 27 would be
2S (USP31). Last, an example of a revision that was officially adopted in USP 32–NF 27 would be  USP32. The following table shows symbols and official dates for Interim Revision Announcements and Supplements to USP 32–NF 27:
USP32. The following table shows symbols and official dates for Interim Revision Announcements and Supplements to USP 32–NF 27:
| USP 32–NF 27
Revision Document |
|||
| Supplement | Interim Revision
Announcement |
Official Date | Symbols |
| 35(1) | Feb. 1, 2009 | ||
| 35(2) | Apr. 1, 2009 | ||
| 35(3) | June 1, 2009 | ||
| 1 | Aug. 1, 2009 | ||
| 35(4) | Aug. 1, 2009 | ||
| 35(5) | Oct. 1, 2009 | ||
| 2 | Dec. 1, 2009 | ||
| 35(6) | Dec. 1, 2009 | ||
Chemical Names and CAS Registry Numbers—
Chemical subtitles given in the monographs are index names used by the Chemical Abstracts Service (CAS) of the American Chemical Society. They are provided only in monographs in which the titles specify substances that are definable chemical entities. The first subtitle is the inverted form of the systematic chemical name developed by CAS. This is presented in accordance with the rules established over the years by the International Union of Pure and Applied Chemistry (IUPAC) and the International Union of Biochemistry, and this form is employed in the current issues of Chemical Abstracts (CA). The second subtitle, given in uninverted form, is of a systematic type formerly used in CA. It is identical with, or closely resembles, the chemical name sanctioned and employed by IUPAC and by the World Health Organization (WHO). IUPAC names make generous use of nonsystematic and semisystematic (often referred to as “trivial”) names and qualifying terms, all of which impede electronic manipulation. In contrast, CAS names are fully systematic for most substances and are amenable to search and retrieval. The two subtitles referred to above are frequently identical, and a CAS synonym is occasionally supplied as a third subtitle. Monographs with chemical subtitles generally also carry CAS registry numbers. These italicized, bracketed numbers function independently of nomenclature as invariant numerical designators of unique, unambiguous chemical substances in the CAS registry and thus are convenient and widely used.
Print and Electronic Presentations—
All USP–NF publications are available in print form. In addition, USP–NF and its two annual Supplements are available in compact disc (CD) and online versions. The CD version makes USP–NF accessible to users on their computer hard drives. The online format allows individual registered users to access the online format through the Internet. Both electronic formats provide access to official USP–NF content, along with extensive search options. The electronic formats are cumulatively updated to integrate the content of Supplements. Searchable electronic versions of PF and of the USP Dictionary also are available.
USP GOVERNANCE, STANDARDS-SETTING, AND ADVISORY BODIES
USP's governing, standards-setting, and advisory bodies include the USP Convention, the Board of Trustees, the Council of Experts and its Expert Committees, Advisory Panels, and staff. Additional volunteer bodies include Stakeholder Forums, Project Teams, and Advisory Groups, which act in an advisory capacity to provide input to USP's governing, standards-setting, and management bodies.
USP Convention—
USP's direction and priorities are determined by more than 400 Convention members divided into nine categories (see the People section). Eligible organizations within each membership category are invited to appoint a representative. Convention composition is determined to ensure suitable representation of those sections of the health care system that are influenced by, and in turn influence, USP's activities. Convention members elect USP's President, Treasurer, and other members of the Board of Trustees as well as the Council of Experts. They also vote on resolutions to guide USP's scientific policy and public health initiatives and update, as needed, USP's Constitution and By-Laws. The next meeting of the USP Convention is scheduled for April 2010 in Washington, DC.
Board of Trustees—
USP's Board of Trustees is entrusted with management of the business affairs, finances, and property of USP. During its five-year term, the Board defines USP's strategic direction through its key policy and operational decisions. A listing of the members of the 2005–2010 Board of Trustees appears in the People section.
Council of Experts—
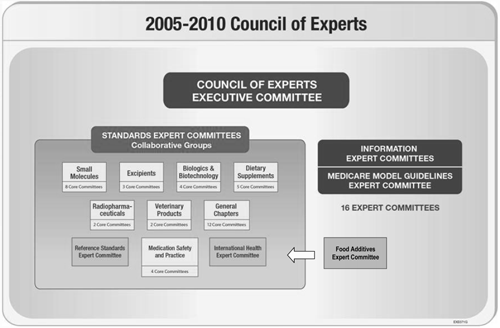
The Council of Experts is the standards-setting body of USP. It is composed of 57 Expert Committee Chairs elected to five-year terms by USP's Convention members. A Nominating Committee, consisting of the Chair of the Council of Experts, the Convention President, and the Vice Chair of the Nominating Committee for the Council of Experts, nominates individuals who are subsequently elected by the members of the Council of Experts to serve as Expert Committee members. Collectively, the Expert Committee Chairs and members comprise more than 500 volunteers drawn from 50 countries. The 41 Standards Expert Committees are responsible for the content of USP–NF, the Food Chemicals Codex, and associated publications (see Figure 1) and organized in Collaborative Groups for topics of common interest. The Information Expert Committees focus on development of Model Guidelines for the Medicare Modernization Act and other information activities. The Executive Committee of the Council of Experts (see the People section) provides overall direction, is an appeals body, and performs other functions that support the Council's operations.
Advisory Panels to the Council of Experts—
The Chair of the Council of Experts may appoint Advisory Panels to assist the Council of Experts in reaching scientific decisions and implementing new USP directives relating to USP–NF. A listing of Advisory Panels is provided in the People section. This list changes frequently as the work of Advisory Panels concludes and new ones start their deliberations. There are more than 350 Advisory Panel members who contribute to the standards-setting activities of the Council of Experts.
Stakeholder Forums and Project Teams—
USP has formed several domestic and international Stakeholder Forums and Project Teams in the 2005–2010 cycle to exchange information and receive comment on USP's standards-setting activities. Depending on the topic, a stakeholder forum may form project teams to work on selected topics. USP has also formed country and regional Stakeholder Forums. The following are lists of Stakeholder Forums for the 2005–2010 cycle.
Domestic Stakeholder Forums
(United States and Canada)
- Prescription/Nonprescription
- Biologics and Biotechnology
- Compounding
- Dietary Supplements
- Food Ingredients
- Patient Safety
International Stakeholder Forums
- Europe
- India
- Latin America
USP also conducts Annual Scientific Meetings in the United States, India, China, Latin America, and the Middle East/North Africa.
Staff—
USP maintains a staff of over 500 scientists, professionals, and administrative personnel at its Rockville, Maryland, headquarters. Additional staff members are located at the account management office in Basel, Switzerland, and laboratory complexes in Hyderabad, India; Shanghai, China; and São Paolo, Brazil.
RULES AND PROCEDURES
Governing Documents—
USP–NF standards are recognized widely because they are authoritative and science-based and are established by a transparent and credible process. See the Articles of Incorporation section in this book; the Constitution and Bylaws and the Rules and Procedures of the 2005–2010 Council of Experts are available on USP's website (www.usp.org). Collectively, these documents serve USP volunteers and staff as the governing principles for USP's standards-setting activities.
Conflicts of Interest—
USP's Conflict of Interest provisions require all members of the Council of Experts, its Expert Committees, Advisory Panels, Board of Trustees, and key staff to disclose significant financial interests in companies or other entities that are subject to USP–NF standards or that may be affected by USP–NF information. Members of the Board of Trustees, Council of Experts, and related bodies are not allowed to vote on any matter in which they have a conflict of interest or the appearance of a conflict of interest.
Confidentiality and Document Disclosure—
Members of the Council of Experts, Expert Committees, and Advisory Panels sign confidentiality agreements, in keeping with the confidentiality provisions of the Rules and Procedures of the Council of Experts. The USP Document Disclosure Policy, available on USP's website, contributes to the transparency of the standards-setting process by making information available to the public, yet provides protection to manufacturers and others who submit confidential information to USP.
Authority for Publication—
USP–NF is published in accordance with Chapter VI, Section 8, of the USP Bylaws, which states, “The Board of Trustees shall authorize the revision and release of text to the United States Pharmacopeia and the National Formulary. Upon approval of the content by the Council of Experts, in accordance with the rules and procedures adopted under Section 9, the Board of Trustees shall then act upon releasing the text and upon designating the date when it is to become official, said date to be reasonably distant from the date of its release. The Executive Vice President–CEO shall, annually or more frequently, upon specific request of the Board of Trustees, certify that the information contained in the United States Pharmacopeia, National Formulary, or other authorized publications has been prepared in accordance with the rules and procedures under Section 9.”
USP–NF REVISION PROCESS
Public Participation—
Although USP's Council of Experts is the ultimate decision-making body for USP–NF standards, these standards are developed by an exceptional process of public involvement and substantial interaction between USP and its stakeholders, both domestically and internationally. Participation in the revision process results from the support of many individuals and groups and also from scientific, technical, and trade organizations.
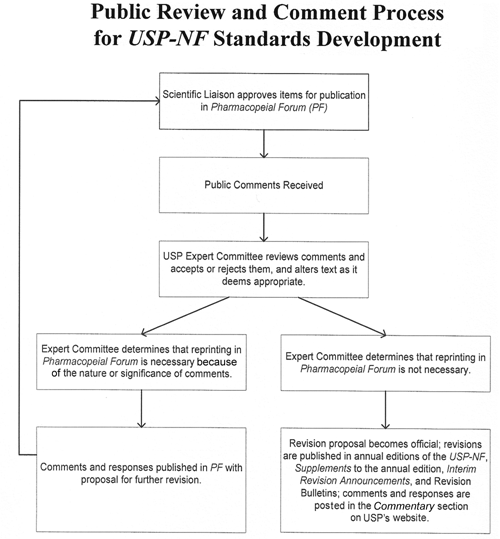
Requests for revision of monographs, either new monographs or those needing updating, contain information submitted voluntarily by manufacturers and other interested parties. At times USP staff may develop information to support a monograph Request for Revision. USP has prepared a document titled Guideline for Submitting Requests for Revision to USP–NF (available at www.usp.org, click on USP–NF). Via PF, USP solicits and encourages public comment on these monographs, General Chapters, and other draft documents. USP scientific liaisons to Expert Committees review these responses and create draft proposals that are provided to the Council of Experts. These drafts become official when Expert Committees ballot to make them official in USP–NF. Thus, the USP standards-setting process gives those who manufacture, regulate, and use therapeutic products the opportunity to comment on the development and revision of USP–NF standards. Because of the voting process and its special link to the U.S. government in law, USP is not considered a voluntary, consensus standards-setting body. Figure 2 shows the public review and comment process and its relationship to standards development.
Working with the Food and Drug Administration (FDA)—
As specified in U.S. law, USP works with the Secretary of the Department of Health and Human Services in many ways. Principal agencies in the Department for this work are the Food and Drug Administration and the Centers for Medicare and Medicaid Services. The FDA Liaison Program allows FDA representatives to participate in Expert Committee meetings, enabling continuing interactions between FDA scientific staff and Expert Committee activities. Staff in the FDA Centers who are responsible for review of compendial activities provide specific links and opportunities for exchange of comments. Mr. Larry A. Ouderkirk in the Center for Drug Evaluation and Research provides a primary compendial link between FDA and USP.
LEGAL RECOGNITION
Recognition of USP–NF—
USP–NF is recognized by law and custom in many countries throughout the world. In the United States, the federal Food, Drug, and Cosmetic Act (FD&C Act) defines the term “official compendium” as the official USP, the official NF, the official Homeopathic Pharmacopeia of the United States, or any supplement to them. FDA may enforce compliance with official standards in USP–NF under the adulteration and misbranding provisions of the FD&C Act. These provisions extend broad authority to FDA to prevent entry to or remove designated products from the United States market on the basis of standards in the USP–NF.
The identity of an official article, as expressed by its name, is established if it conforms in all respects to the requirements of its monograph and other relevant portions of the compendia. The FD&C Act stipulates that an article may differ in strength, quality, or purity and still have the same name if the difference is stated on the article's label. FDA requires that names for articles that are not official must be clearly distinguishing and differentiating from any name recognized in an official compendium. Official preparations (a drug product, a dietary supplement including nutritional supplements, or a finished device) may contain additional suitable ingredients. (See General Notices.)
Drugs—
USP's goal is to have substance and preparation (product) monographs in USP–NF for all FDA-approved drugs, including biologics, and their ingredients. USP also develops monographs for therapeutic products not approved by FDA, e.g., pre-1938 drugs, dietary supplements, and compounded preparations. Although submission of information needed to develop a monograph by the Council of Experts is voluntary, compliance with a USP–NF monograph, if available, is mandatory.
Biologics—
In the United States, although some biologics are regulated under the provisions of the Public Health Service Act (PHSA), provisions of the FD&C Act also apply to these products. For this reason, products approved under the PHSA should comply with the adulteration and misbranding provisions of the FD&C Act at Section 501(b) and 502(g) and, thus, should conform to applicable official monographs in USP–NF.
Medical Devices—
Section 201(h) of the FD&C Act defines a device as an instrument, apparatus, similar article, or component thereof recognized in USP–NF. Section 502(e) of the FD&C Act defines the established name of a device in the absence of an FDA designation of the official name as the official title in an official compendium. Despite these statutory provisions, there is no comparable recognition of USP's standards-setting authority and ability to define a medical device as exists for other FDA-regulated therapeutic products. Under authority granted by the Food and Drug Administration Modernization Act of 1997, the Center for Devices and Radiological Health recognizes national and international standards, including some USP tests and assays, for medical devices.
Dietary Supplements—
The Dietary Supplement Health and Education Act of 1994 amendments to the FD&C Act name USP and NF as the official compendia for dietary supplements. The amendments also provide that a dietary supplement may be deemed misbranded if it is covered by a monograph in an official compendium, is represented as conforming to this monograph, but fails to conform. The dietary supplement must be represented as conforming to a USP–NF dietary supplement monograph in order for the compendial standards to apply. This contrasts with pharmaceutical products, wherein conformance to the monograph is mandatory whether or not the product claims to conform.
Compounded Preparations—
Preparation monographs provide information or standards applicable in compounding. Compounding means the preparation, mixing, assembling, packaging, or labeling of a drug or device or other article, as the result of a practitioner's order or in anticipation of such an order based on routine, regularly observed prescribing patterns. Standards in USP–NF for compounded preparations may be enforced at both the state and federal levels, e.g., if a practitioner writes a prescription for a compounded preparation that is named in a USP–NF monograph, the preparation, when tested, must conform to the stipulations of the monograph so named.
Nomenclature—
In the United States, FDA has authority to establish names for drug products and ingredients and to determine proper names for biologics. In most cases, however, FDA works with the United States Adopted Names (USAN) Council to determine names for drug and biological substances and with USP to determine drug product names. Oversight of proprietary names and proper names is the responsibility of FDA, working with applicants.
The USAN Council's program began in 1961 by providing ingredient names for drugs prior to their marketing. USP participates in this activity, together with the American Medical Association, the American Pharmacists Association, and FDA. The Council's output is incorporated, along with other names for drugs (including generic, proprietary, and chemical names and code designations), in the USP Dictionary of USAN and International Drug Names. Since 1988 this publication has been recognized by federal regulation as the source of established names for drug substances in the United States.
Drug product names can be established by FDA, but more often are developed cooperatively by FDA and the USP Council of Experts' Nomenclature Expert Committee. The names developed by this Expert Committee are used as the titles of the relevant monographs, and as such are recognized as the “established names” for drug products under section 502(e)(3) of the FD&C Act. The USP Drug Nomenclature Committee was formed in 1986 to supplement the Executive Committees of the Drug Standards Division and the Information Division and to prevent any inconsistency regarding nomenclature. Following the 2000 meeting of the USP Convention, the responsibilities for devising and, when necessary, revising labeling requirements were delegated to this Expert Committee, which is now named the Nomenclature Expert Committee. The Expert Committee's work does not overlap that of the USAN Council. Rather, it is complementary and is concerned with standardization of compendial names, particularly dosage form names, and names for combination drug products.
HARMONIZATION ACTIVITIES
Pharmacopeial Discussion Group—
USP harmonizes pharmacopeial excipient monographs and General Chapters through the Pharmacopeial Discussion Group (PDG), which includes representatives from the European, Japanese, and United States pharmacopeias, and WHO (as an observer). According to the PDG definition, “a pharmacopeial general chapter or other pharmacopeial document is harmonized when a pharmaceutical substance or product tested by the document's harmonized procedure yields the same results, and the same accept/reject decision is reached.” General Information Chapter  1196
1196 , Pharmacopeial Harmonization, provides (1) the PDG Policy Statement, (2) the PDG Working Procedures and a definition of each stage of harmonization, (3) a discussion, (4) a status report, and (5) a glossary.
, Pharmacopeial Harmonization, provides (1) the PDG Policy Statement, (2) the PDG Working Procedures and a definition of each stage of harmonization, (3) a discussion, (4) a status report, and (5) a glossary.
OTHER USP–NF RELATED PUBLICATIONS
Chromatographic Reagents—
This comprehensive reference provides detailed information needed to conduct chromatographic procedures found in USP–NF. Chromatographic Reagents lists the brand names of the column reagents cited in every proposal for new or revised gas- or liquid-chromatographic analytical procedures that have been published in PF since 1980. Chromatographic Reagents also helps to track which column reagents were used to validate analytical procedures that have become official. The branded column reagents list is updated bimonthly in PF.
USP Pharmacists' Pharmacopeia—
USP–NF is directed primarily to pharmaceutical and dietary supplement manufacturers, although it contains many monographs and allied text useful for compounding practitioners. To better accommodate the needs of these practitioners and more generally the needs of the pharmacy community, USP has made available the USP Pharmacists' Pharmacopeia. This text provides pharmacy-relevant abridged official text from the USP–NF as well as authorized information. The former refers to standards for official articles; the latter is more general information designed to be useful to practitioners. Both types of text are developed under the Rules and Procedures of the Council of Experts. The USP Pharmacists' Pharmacopeia is available both in print and in a web-based version.
USP Dictionary—
The USP Dictionary of USAN and International Drug Names provides in a single volume the most up-to-date United States Adopted Names of drugs; official USP–NF names; nonproprietary, brand, and chemical names; graphic formulas; molecular formulas and weights; CAS registry numbers and code designations; drug manufacturers; and pharmacologic and therapeutic categories. The Dictionary helps to ensure the accuracy of the following: product labeling; reports, articles, and correspondence; FDA regulatory filings; and pharmaceutical package inserts. It is published annually (latest edition April 2008) and is recognized by FDA as the official source for established drug names (See Nomenclature section.)
USP Catalog—
When referenced in a compendial procedure, use of official USP–NF Reference Standards promotes uniform quality of drugs and supports first-, second-, and third-party testing of all manufactured and compounded articles. The publication listing the collection of official USP–NF Reference Standards can be accessed on the USP website at www.usp.org and is available in print form by contacting USP Sales and Marketing staff at 301-816-8237. The listing identifies new items, replacement lots, lots of a single item that are simultaneously official, lots deleted from official status, and a preview of items eventually to be adopted. Purchase order information is included, and the names of distributors who can facilitate international availability of these items are suggested. This program benefits from the widespread voluntary contribution of suitable materials and test data from pharmaceutical manufacturers. USP advances this unofficial material to official status via careful characterization studies and collaborative testing, followed by review and, if appropriate, approval by the Reference Standards Committee of the Council of Experts.