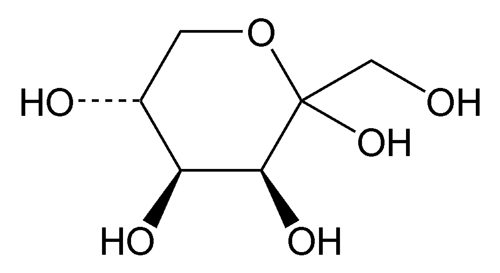
Tagatose
» Tagatose is a ketohexose, an epimer of d-fructose inverted at C-4. It is obtained from d-galactose by isomerization under alkaline conditions in the presence of calcium. It contains not less than 98.0 percent of C6H12O6, calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers, and store at room temperature.
Identification—
A:
The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay.
B:
It meets the requirements of the test for Specific rotation  781S
781S .
.
C:
Add 3 mL of a solution (1 in 5) to 5 mL of hot alkaline cupric tartrate TS: a copious red precipitate of cuprous oxide is formed.
Microbial enumeration tests  61
61 and Tests for specified microorganisms
and Tests for specified microorganisms  62
62 —
It meets the requirements of the tests for absence of Salmonella species and Escherichia coli. The total aerobic microbial count does not exceed 1000 cfu per g, and the total combined molds and yeasts count does not exceed 100 cfu per g.
—
It meets the requirements of the tests for absence of Salmonella species and Escherichia coli. The total aerobic microbial count does not exceed 1000 cfu per g, and the total combined molds and yeasts count does not exceed 100 cfu per g.
Loss on drying  731
731 —
Dry it at 102
—
Dry it at 102 for 2 hours: it loses not more than 0.5% of its weight.
for 2 hours: it loses not more than 0.5% of its weight.
Total ash  561
561 :
not more than 0.1%, determined on a 1.0-g specimen.
:
not more than 0.1%, determined on a 1.0-g specimen.
Limit of lead—
Test solution—
Accurately weigh about 2.5 g of Tagatose, and dissolve in a mixture of 4 mL of sulfuric acid and 5 mL of hydrochloric acid. Transfer the solution to a 50-mL volumetric flask, dilute with water to volume, and mix.
Standard lead solution—
Dissolve 1.60 g of lead nitrate in diluted nitric acid (10 mL of nitric acid diluted with 20 mL water, boiled to remove nitrous fumes, and cooled), and dilute with water to 1000 mL. Dilute 10.0 mL of this solution with water to 500 mL. This solution contains the equivalent of 20 µg of lead per mL.
Calibration solutions—
To a series of 100-mL volumetric flasks, pipet 0, 1, 2, 3, 4, and 5 mL of the Standard lead solution, and dilute with water to about 50 mL. Add 8 mL of sulfuric acid and 10 mL of hydrochloric acid to each flask, shake to dissolve, and dilute with water to volume. These solutions contain 0, 0.2, 0.4, 0.6, 0.8, and 1.0 µg of lead per mL.
Procedure—
Concomitantly determine the absorbances of the Calibration solutions and the Test solution at the wavelength of maximum absorbance at 283.3 nm, with a suitable atomic absorption spectrophotometer (see Spectrophotometry and Light-Scattering  851
851 ). Plot the absorbances of the Calibration solutions versus the concentration of lead. Using this graph, determine the concentration of lead in the Test solution. Not more than 1 µg per g is found.
). Plot the absorbances of the Calibration solutions versus the concentration of lead. Using this graph, determine the concentration of lead in the Test solution. Not more than 1 µg per g is found.
Assay—
Mobile phase—
Prepare a solution in water containing 50 mg of calcium acetate per L.
Standard preparation—
Dissolve an accurately weighed quantity of USP Tagatose RS in water to obtain a solution having a known concentration of about 5 mg per mL. Pass through a 0.2-µm filter.
Assay preparation—
Transfer about 50 mg of Tagatose, previously dried, to a 10-mL volumetric flask, and dissolve in about 8 mL of water. Dilute with water to volume, and pass through a 0.2-µm filter.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a refractive index detector and a 7.8-mm × 30-cm column that contains 9-µm packing L19. The column temperature is maintained at 85
)—
The liquid chromatograph is equipped with a refractive index detector and a 7.8-mm × 30-cm column that contains 9-µm packing L19. The column temperature is maintained at 85 . The flow rate is about 0.6 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 2.0%.
. The flow rate is about 0.6 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 20 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of C6H12O6 in the portion of Tagatose taken by the formula:
10C(rU / rS)
in which C is the concentration, in mg per mL, of USP Tagatose RS in the Standard preparation; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Robert H. Lafaver, B.A.
Scientist 1-301-816-8335 |
(EM105) Excipient Monographs 1 |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
|
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance | |
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance |
USP32–NF27 Page 1367
Pharmacopeial Forum: Volume No. 31(3) Page 819
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.