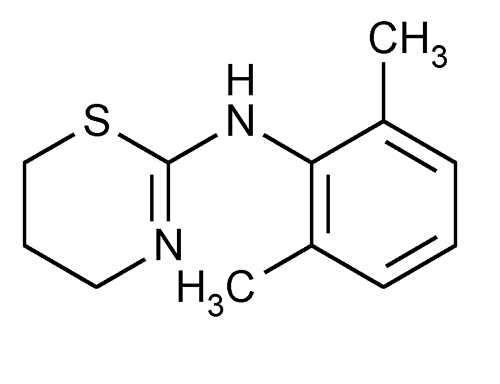
Xylazine
» Xylazine contains not less than 98.0 percent and not more than 102.0 percent of C12H16N2S.
Packaging and storage—
Preserve in tight containers. Store at 25 , excursions permitted between 15
, excursions permitted between 15 and 30
and 30 .
.
Labeling—
Where it is intended for veterinary use only, the label so states.
Identification—
C: Thin-Layer Chromatographic Identification Test  201
201 —
—
Test solution:
2 mg per mL, in chloroform.
Developing solvent system:
acetone, chloroform, and methanol (2:1:1).
Procedure—
Prior to the applications of the Test solution and the Standard solution, dry the plate at 105 for not less than 30 minutes, and allow it to cool in a desiccator. Allow the applications to dry with the aid of a current of warm air, and develop. Examine under short-wavelength UV light: the size, intensity, and RF value of the principal spot obtained from the Test solution correspond to those of the principal spot obtained from the Standard solution.
for not less than 30 minutes, and allow it to cool in a desiccator. Allow the applications to dry with the aid of a current of warm air, and develop. Examine under short-wavelength UV light: the size, intensity, and RF value of the principal spot obtained from the Test solution correspond to those of the principal spot obtained from the Standard solution.
Loss on drying  731
731 —
Dry it in vacuum at 60
—
Dry it in vacuum at 60 for 4 hours: it loses not more than 0.5% of its weight.
for 4 hours: it loses not more than 0.5% of its weight.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Heavy metals, Method II  231
231 :
20 µg per g.
:
20 µg per g.
Limit of 3-amino-1-propanol—
Prepare a test solution of Xylazine in methanol containing 100 mg per mL, using sonication to achieve dissolution. Prepare a Standard solution of 3-amino-1-propanol in methanol containing 0.5 mg per mL. Separately apply 5 µL of the test solution and the Standard solution to a thin-layer chromatographic plate (see Chromatography  621
621 ) coated with a 0.25-mm layer of chromatographic silica gel. Allow the applications to dry, and develop the chromatograms in a saturated chromatographic chamber, containing a solvent system consisting of a mixture of alcohol and ammonium hydroxide (80:20) until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the chromatographic chamber, mark the solvent front, and air-dry the plate. Spray the plate with an alcoholic solution of ninhydrin (1 in 500), and immediately heat the plate in an oven at 105
) coated with a 0.25-mm layer of chromatographic silica gel. Allow the applications to dry, and develop the chromatograms in a saturated chromatographic chamber, containing a solvent system consisting of a mixture of alcohol and ammonium hydroxide (80:20) until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the chromatographic chamber, mark the solvent front, and air-dry the plate. Spray the plate with an alcoholic solution of ninhydrin (1 in 500), and immediately heat the plate in an oven at 105 . When the spots are visible, remove the plate from the oven, and allow to cool. Examine the chromatograms, and compare the intensities of the spots corresponding to 3-amino-1-propanol: the intensity of the spot for 3-amino-1-propanol obtained from the test solution is not greater than that of the spot for 3-amino-1-propanol obtained from the Standard solution (0.5%).
. When the spots are visible, remove the plate from the oven, and allow to cool. Examine the chromatograms, and compare the intensities of the spots corresponding to 3-amino-1-propanol: the intensity of the spot for 3-amino-1-propanol obtained from the test solution is not greater than that of the spot for 3-amino-1-propanol obtained from the Standard solution (0.5%).
Limit of acetone and isopropyl alcohol—
Diluent—
Dilute 15 mL of glacial acetic acid with water to 1000 mL, and mix.
Standard solution—
Transfer 10.0 µL each of acetone and isopropyl alcohol to a 500-mL volumetric flask, dilute with Diluent to volume, and mix. This solution contains 15.8 µg of acetone per mL and 15.7 µg of isopropyl alcohol per mL.
Test solution—
Transfer about 100 mg of Xylazine, accurately weighed, to a 10-mL volumetric flask, dissolve in and dilute with Diluent to volume, and mix.
Chromatographic system (see Chromatography  621
621 )—
The gas chromatograph is equipped with a flame-ionization detector and a 2-mm × 1.8-m column packed with 0.1% phase G25 on 80- to 100-mesh support S7. Helium is used as the carrier gas with a flow rate of about 30 mL per minute. The injection port and detector temperatures are maintained at about 240
)—
The gas chromatograph is equipped with a flame-ionization detector and a 2-mm × 1.8-m column packed with 0.1% phase G25 on 80- to 100-mesh support S7. Helium is used as the carrier gas with a flow rate of about 30 mL per minute. The injection port and detector temperatures are maintained at about 240 and 275
and 275 , respectively. The system is programmed according to the following steps. The column temperature is maintained at 30
, respectively. The system is programmed according to the following steps. The column temperature is maintained at 30 for 6 minutes after each injection, then increased to 100
for 6 minutes after each injection, then increased to 100 at a rate of 10
at a rate of 10 per minute, then increased further to 220
per minute, then increased further to 220 at a rate of 15
at a rate of 15 per minute, and maintained for 10 minutes. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.75 for acetone and 1.0 for isopropyl alcohol; the resolution, R, between acetone and isopropyl alcohol is not less than 2.0; the tailing factor determined from each analyte peak is not more than 2.0; and the relative standard deviation for replicate injections is not more than 2.0%.
per minute, and maintained for 10 minutes. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.75 for acetone and 1.0 for isopropyl alcohol; the resolution, R, between acetone and isopropyl alcohol is not less than 2.0; the tailing factor determined from each analyte peak is not more than 2.0; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 2 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the areas for the major peaks. Calculate the percentages of acetone and isopropyl alcohol in the portion of Xylazine taken by the formula:
(C/W)(rU / rS)
in which C is the concentration, in µg per mL, of acetone or isopropyl alcohol in each mL of the Standard solution; W is the weight, in mg, of Xylazine taken to prepare the Test solution; and rU and rS are the responses for the relevant analyte peak obtained from the Test solution and the Standard solution, respectively: not more than 0.02% of acetone and not more than 0.2% of isopropyl alcohol are found.
Chromatographic purity—
Solution A, Solution B, Mobile phase, and Diluent—
Proceed as directed in the Assay.
Standard solution—
Quantitatively dilute an accurately measured volume of the Standard preparation prepared in the Assay with Diluent to obtain a solution having a concentration of 0.008 mg of USP Xylazine RS per mL.
Test solution—
Transfer about 100 mg of Xylazine, accurately weighed, to a 10-mL volumetric flask, add 5.0 mL of Solution B, and swirl to dissolve. Add about 4 mL of Solution A, and swirl. Dilute with Solution A to volume, and mix.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 205-nm detector and a 4.6-mm × 25-cm column that contains packing L7 and a guard column. The flow rate is about 1 mL per minute. Equilibrate the column with a mobile phase consisting of 75% Solution A and 25% Solution B. Maintain this composition for 8 minutes following each injection, after which the proportion of Solution B is increased linearly from 25% to 70% over a period of 27 minutes, and maintained at that composition for 5 minutes; then rapidly increase the proportion of Solution A to 75% before the next injection. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the tailing factor is not more than 1.5; and the relative standard deviation for replicate injections is not more than 5.0%.
)—
The liquid chromatograph is equipped with a 205-nm detector and a 4.6-mm × 25-cm column that contains packing L7 and a guard column. The flow rate is about 1 mL per minute. Equilibrate the column with a mobile phase consisting of 75% Solution A and 25% Solution B. Maintain this composition for 8 minutes following each injection, after which the proportion of Solution B is increased linearly from 25% to 70% over a period of 27 minutes, and maintained at that composition for 5 minutes; then rapidly increase the proportion of Solution A to 75% before the next injection. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the tailing factor is not more than 1.5; and the relative standard deviation for replicate injections is not more than 5.0%.
Procedure—
Separately inject equal volumes (about 10 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the areas for the major peaks. Calculate the percentage of each impurity in the Xylazine taken by the formula:
1000(C/W)(ri F/rS)
in which C is the concentration, in mg per mL, of USP Xylazine RS in the Standard solution; W is the weight, in mg, of Xylazine taken to prepare the Test solution; ri is the response of any individual impurity peak in the chromatogram of the Test solution that is not present in the chromatogram of the Diluent; F is the response factor of 0.72 for the 2,6-dimethylaniline peak at a response time of about 0.8 relative to the retention time of xylazine, of 0.36 for an impurity at a relative retention time of about 1.3, 0.37 for 2,6-dimethylphenyl isothiocyanate at a relative retention time of about 2, and 1.0 for any other impurity; and rS is the response of the xylazine peak in the chromatogram of the Standard solution: not more than 0.5% of any individual impurity is found; and the sum of all impurities found is not more than 1%.
Assay—
Solution A—
Dissolve 3.03 g of sodium 1-heptanesulfonate in 800 mL of water, adjust with 2 N sulfuric acid to a pH of 3.0, dilute with water to 1000 mL, and mix. Pass through a filter having a 0.5-µm or finer porosity.
Solution B—
Use acetonitrile.
Mobile phase—
Use variable mixtures of Solution A and Solution B as directed for Chromatographic system.
Diluent—
Prepare a mixture of Solution A and Solution B (50:50).
Standard preparation—
Prepare a solution of USP Xylazine RS in Diluent having a known concentration of about 0.4 mg per mL.
Assay preparation—
Transfer about 10 mg of Xylazine, accurately weighed, to a 25-mL volumetric flask, dilute with Diluent to volume, and mix.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 226-nm detector and a 3.9-mm × 30-cm column that contains packing L1. The flow rate is about 1 mL per minute. Equilibrate the column with a mobile phase consisting of 70% Solution A and 30% Solution B. Maintain this composition for 5 minutes following each injection, after which the proportion of Solution B is increased linearly from 30% to 40% over a period of 5 minutes, and maintained at that composition for 5 minutes; then rapidly increase the proportion of Solution A to 70% before the next injection. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the tailing factor is not more than 2.0; and the relative standard deviation for replicate injections is not more than 2.0%.
)—
The liquid chromatograph is equipped with a 226-nm detector and a 3.9-mm × 30-cm column that contains packing L1. The flow rate is about 1 mL per minute. Equilibrate the column with a mobile phase consisting of 70% Solution A and 30% Solution B. Maintain this composition for 5 minutes following each injection, after which the proportion of Solution B is increased linearly from 30% to 40% over a period of 5 minutes, and maintained at that composition for 5 minutes; then rapidly increase the proportion of Solution A to 70% before the next injection. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the tailing factor is not more than 2.0; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 10 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the areas for the major peaks. Calculate the quantity, in mg, of C12H16N2S in the portion of Xylazine taken by the formula:
25C(rU / rS)
in which C is the concentration, in mg per mL, of USP Xylazine RS in the Standard preparation; and rU and rS are the xylazine peak responses obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Ian DeVeau, Ph.D.
Director, Veterinary Drugs and Radiopharmaceuticals 1-301-816-8178 |
(VET05) Veterinary Drugs 05 |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 3878
Pharmacopeial Forum: Volume No. 29(6) Page 2004
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.