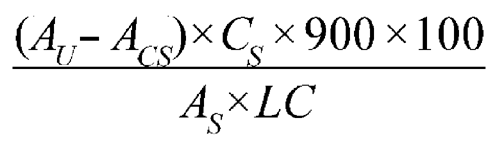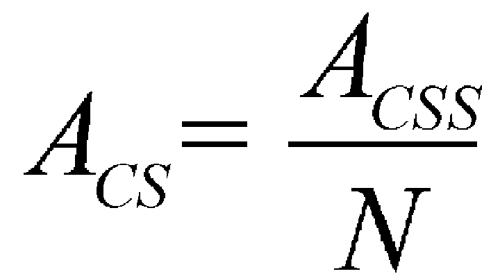Acitretin Capsules
» Acitretin Capsules contain not less than 90.0 percent and not more than 110.0 percent of the labeled amount of acitretin (C21H26O3).
[Caution—Acitretin is a teratogen. Great care should be taken when handling to avoid inhalation of dust or contact with skin.
]
note—Use low-actinic glassware and perform all tests under yellow and subdued light. Make all injections within 1 hour of sample preparation.
Packaging and storage—
Preserve in well-closed, light-resistant containers.
Thin-layer chromatographic identification test  201
201 —
—
Test solution—
Weigh a portion of Capsules, equivalent to 20 mg of acitretin. Grind to a fine powder, then triturate for 30 seconds with 2 mL of tetrahydrofuran. Transfer the suspension to a 12-mL conical centrifuge tube, and centrifuge to obtain a clear supernatant.
Standard solution—
Dissolve an accurately weighed quantity of USP Acitretin RS in tetrahydrofuran to obtain a solution containing about 10 mg per mL.
Application volume:
10 µL.
Developing solvent system:
a mixture of chloroform and methanol (80:20).
Procedure—
Proceed as directed in the chapter, and then air-dry. Spray the plate with a saturated solution of antimony trichloride in chloroform (25 g in 100 mL) followed by concentrated sulfuric acid, and then locate the spots.
Dissolution  711
711 —
—
Medium:
3% sodium lauryl sulfate in deaerated water, pH 9.6 to 10.0; 900 mL.
Apparatus 1:
100 rpm.
Time:
30 minutes.
Determine the amount of acitretin (C21H26O3) dissolved employing the following method.
Standard solution—
Transfer about 14 mg of USP Acitretin RS, accurately weighed, to a 500-mL volumetric flask. Dissolve in 50 mL of alcohol, and dilute with Medium to volume. For Capsules labeled to contain 10 mg, transfer 20.0 mL of this solution to a 50-mL volumetric flask, and dilute with Medium to volume.
Capsule shell preparation—
Dissolve 6 clean empty-shell Capsules in 900 mL of Medium.
Test solution—
Use portions of the solution under test passed through a suitable 0.45-µm filter.
Procedure—
Determine the amount of C21H26O3 dissolved by employing UV absorption at the wavelength of maximum absorbance at about 347 nm, using 2-mm cells, in comparison with the appropriate Standard solution. Use the Medium as a blank. Determine the absorbance of the Capsule shell preparation under the same conditions. Calculate the amount of acitretin (C21H26O3) dissolved by the following formula:
in which AU is the absorbance of the Test solution; ACS is the Capsule shell correction, calculated as shown below; CS is the concentration, in mg per mL, of the appropriate Standard solution; 900 is the volume, in mL, of Medium; 100 is the conversion factor to percentage; AS is the absorbance of the appropriate Standard solution; and LC is the Capsule label claim, in mg. The Capsule shell correction, ACS, is calculated using the following formula:
in which ACSS is the absorbance of the Capsule shell preparation; and N is the number of Capsule shells used to prepare the Capsule shell preparation.
Tolerances—
Not less than 85% (Q) of the labeled amount of acitretin (C21H26O3) is dissolved in 30 minutes.
Uniformity of dosage units  905
905 :
meet the requirements.
:
meet the requirements.
Limit of degradation products—
Diluent, Mobile phase, System suitability solution, and Chromatographic system—
Proceed as directed in the Assay.
Test solution—
Use the Assay preparation.
Procedure—
Inject a volume (about 25 µL) of the Test solution into the chromatograph, record the chromatogram, and measure the peak responses. Calculate the percentage of each degradation product in the portion of Capsules taken by the formula:
100(ri / rs)
in which ri is the peak response for each individual impurity; and rs is the sum of the responses of all of the peaks: not more than 0.5% of acitretin related compound A, not more than 0.4% of any individual unspecified impurity, and not more than 0.8% of total unspecified impurities is found.
Assay—
Diluent—
Prepare a suitable mixture of methanol and tetrahydrofuran (13:10).
Mobile phase—
Prepare a filtered and degassed mixture of methanol, water, alcohol, and glacial acetic acid (74:21:5:0.5). Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard preparation—
In a 100-mL volumetric flask, dissolve about 10 mg of USP Acitretin RS, accurately weighed, in 80 mL of Diluent, and sonicate for 5 minutes. Add 8 mL of water, and quantitatively dilute with Diluent to obtain a solution having a concentration of about 0.1 mg per mL.
System suitability solution—
Transfer 2 mL of the Standard preparation to a clear 4-mL glass vial. After sealing the vial with a teflon-lined silicone septum and cap, place the vial on its side in a light chamber, expose it to 400 foot-candles of fluorescent light for 5 minutes, and then completely wrap the vial with aluminum foil. [note—Exposure to the fluorescent light allows for the formation of two degradation products: acitretin related compound A and the 9-cis isomer [(E,E,Z,E)-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoic acid].]
Assay preparation—
Carefully separate and place both halves of 10 Capsules into a 100-mL volumetric flask. Stopper and shake the flask to remove the fill. Add 8 mL of water while rinsing any fill from the neck of the flask. Place the flask in a water bath set at 45 for 10 minutes, shaking initially and at 5-minute intervals up to 10 minutes. Place the resulting suspension in an ultrasonic bath for 15 minutes. Dilute with Diluent to volume, and sonicate for 5 additional minutes. Cool to room temperature and, if necessary, dilute with Diluent to volume. Filter the suspension, and use the clear filtrate.
for 10 minutes, shaking initially and at 5-minute intervals up to 10 minutes. Place the resulting suspension in an ultrasonic bath for 15 minutes. Dilute with Diluent to volume, and sonicate for 5 additional minutes. Cool to room temperature and, if necessary, dilute with Diluent to volume. Filter the suspension, and use the clear filtrate.
Chromatographic system
(see Chromatography  621
621 )—The liquid chromatograph is equipped with a 365-nm detector and a 4.6-mm × 15-cm column that contains 5-µm L1 packing. The flow rate is about 1.0 mL per minute. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the resolution, R, between acitretin related compound A (relative retention time of about 0.84) and acitretin is not less than 3.0; the resolution, R, between the 9-cis isomer (relative retention time of about 1.09) and acitretin is not less than 1.8. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections of acitretin is not more than 2.0%.
)—The liquid chromatograph is equipped with a 365-nm detector and a 4.6-mm × 15-cm column that contains 5-µm L1 packing. The flow rate is about 1.0 mL per minute. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the resolution, R, between acitretin related compound A (relative retention time of about 0.84) and acitretin is not less than 3.0; the resolution, R, between the 9-cis isomer (relative retention time of about 1.09) and acitretin is not less than 1.8. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections of acitretin is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 25 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of acitretin (C21H26O3) in the portion of Capsules taken by the formula:
100C(rU / rS)
in which C is the concentration, in mg per mL, of USP Acitretin RS in the Standard preparation; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Feiwen Mao, M.S.
Scientist 1-301-816-8320 |
(MDOOD05) Monograph Development-Ophthalmics Oncologics and Dermatologicals |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
|
| Margareth R.C. Marques, Ph.D.
Senior Scientist 1-301-816-8106 |
(BPC05) Biopharmaceutics05 |
USP32–NF27 Page 1426
Pharmacopeial Forum: Volume No. 33(3) Page 392
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.