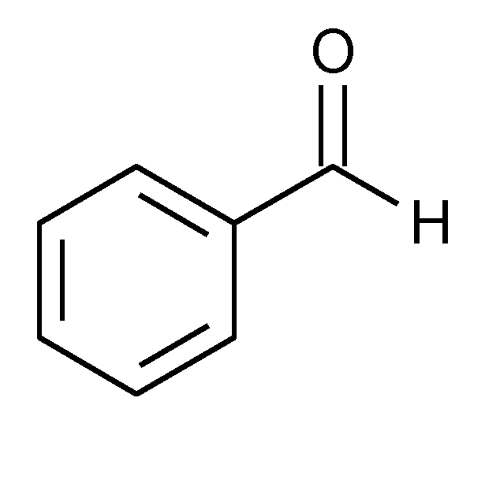
Benzaldehyde
» Benzaldehyde contains not less than 98.0 percent and not more than 100.5 percent of C7H6O.
Packaging and storage—
Preserve in well-filled, tight, light-resistant containers.
Specific gravity  841
841 :
between 1.041 and 1.046 at 25
:
between 1.041 and 1.046 at 25 .
.
Refractive index  831
831 :
between 1.544 and 1.546 at 20
:
between 1.544 and 1.546 at 20 .
.
Limit of hydrocyanic acid—
Shake 0.5 mL of it with 5 mL of water, add 0.5 mL of 1 N sodium hydroxide and 0.1 mL of ferrous sulfate TS, and warm the mixture gently. Upon the addition of a slight excess of hydrochloric acid, no greenish blue color or blue precipitate is produced within 15 minutes.
Limit of nitrobenzene—
Dissolve 1 mL of it in 20 mL of alcohol, and mix with 10 mL of water. Add 1-g portions of zinc and 1-mL portions of 2 N sulfuric acid, as needed, to maintain a brisk evolution of hydrogen for 1 hour. Filter, evaporate the liquid to about 20 mL, and boil 10 mL of the concentrated liquid with 1 drop of potassium dichromate TS: no purplish color is produced.
Chlorinated compounds—
Wind a strip of 20-mesh copper gauze 1.5 cm wide and 5 cm long around the end of a copper wire. Heat the gauze in the nonluminous flame of a Bunsen burner until it glows without coloring the flame green. Permit the gauze to cool, and heat several times until a thick coat of oxide has formed. With a medicine dropper, apply 2 drops of Benzaldehyde to the cooled gauze, ignite, and permit it to burn freely in the air. Again cool the gauze, add 2 more drops of it, and burn as before. Repeat this process until a total of 6 drops has been added and ignited. Then hold the gauze in the outer edge of the Bunsen flame, adjusted to a height of about 4 cm: not even a transient green color is imparted to the flame.
Assay—
Transfer about 1 mL of Benzaldehyde to a tared, glass-stoppered weighing bottle, and weigh accurately. Loosen the stopper, and transfer the weighing bottle and contents to a 250-mL conical flask containing 25 mL of a reagent prepared by dissolving 34.7 g of hydroxylamine hydrochloride in 160 mL of water and diluting this solution with alcohol to 1000 mL. Rinse down the sides of the flask with 50 mL of the reagent. Allow to stand for 10 minutes, add 1 mL of bromophenol blue TS, and titrate the liberated hydrochloric acid with 1 N sodium hydroxide VS to a light-green endpoint. Perform a blank determination (see Residual Titrations under Titrimetry  541
541 ). Each mL of 1 N sodium hydroxide is equivalent to 106.1 mg of C7H6O.
). Each mL of 1 N sodium hydroxide is equivalent to 106.1 mg of C7H6O.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Robert H. Lafaver, B.A.
Scientist 1-301-816-8335 |
(EM105) Excipient Monographs 1 |
USP32–NF27 Page 1172
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.