Bacteriostatic Sodium Chloride Injection
» Bacteriostatic Sodium Chloride Injection is a sterile, isotonic solution of Sodium Chloride in Water for Injection, and it contains one or more suitable antimicrobial agents. It contains not less than 0.85 percent and not more than 0.95 percent of NaCl.
note—Use Bacteriostatic Sodium Chloride Injection with due regard for the compatibility of the antimicrobial agent or agents it contains with the particular medicinal substance that is to be dissolved or diluted.
Packaging and storage—
Preserve in single-dose or multiple-dose containers, of not larger than 30-mL size, preferably of Type I or Type II glass.
Labeling—
Label it to indicate the name(s) and proportion(s) of the added antimicrobial agent(s). Label it also to include the statement “not for use in newborns” in boldface capital letters, on the label immediately under the official name, printed in a contrasting color, preferably red. Alternatively, the statement may be placed prominently elsewhere on the label if the statement is enclosed within a box. Label it also to include the statement “not for inhalation.”
Antimicrobial agent(s)—
It meets the requirements under Antimicrobial Preservatives—Effectiveness  51
51 , and meets the labeled claim for content of the antimicrobial agent(s) as determined by the method set forth under Antimicrobial Agents—Content
, and meets the labeled claim for content of the antimicrobial agent(s) as determined by the method set forth under Antimicrobial Agents—Content  341
341 , except to use the following procedure when methylparaben and propylparaben are used as the antimicrobial agents.
, except to use the following procedure when methylparaben and propylparaben are used as the antimicrobial agents.
Mobile phase—
Prepare a filtered and degassed mixture of methanol and water (70:30). Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard preparation—
Dissolve accurately weighed quantities of USP Methylparaben RS and USP Propylparaben RS in methanol, and dilute quantitatively, and stepwise if necessary, with methanol to obtain a solution having known concentrations of about 1.2 and 0.12 mg per mL, respectively. Pipet 5 mL of this solution into a 50-mL volumetric flask, add by pipet 30 mL of methanol, dilute with water to volume, and mix.
Test preparation—
Pipet 1 mL of Injection into a 10-mL volumetric flask, add by pipet 7 mL of methanol, dilute with water to volume, and mix.
Chromatographic system
(see Chromatography  621
621 )—The liquid chromatograph is equipped with a 254-nm detector and a 4-mm × 30-cm column that contains packing L1. The flow rate is about 1.5 mL per minute. Chromatograph the Standard preparation as directed for Procedure: the capacity factor, k¢, is 0.52 for methylparaben and 1.05 for propylparaben, with a minimum separation factor (
)—The liquid chromatograph is equipped with a 254-nm detector and a 4-mm × 30-cm column that contains packing L1. The flow rate is about 1.5 mL per minute. Chromatograph the Standard preparation as directed for Procedure: the capacity factor, k¢, is 0.52 for methylparaben and 1.05 for propylparaben, with a minimum separation factor (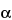 ) of about 2.0.
) of about 2.0.
Procedure—
Separately inject equal volumes (about 12 µL) of the Standard preparation and the Test preparation into the chromatograph by means of a suitable microsyringe or sampling valve, adjusting the specimen size and other operating parameters such that the peak obtained with the Standard preparation is about 0.7 full scale. Record the chromatograms, and measure the height of the peaks, at identical retention times, obtained with the Test preparation and the Standard preparation, and calculate the concentration in mg per mL, in the portion of methylparaben or propylparaben taken by the formula:
C(HU / HS)
in which C is the concentration, in mg per mL, of USP Methylparaben RS or USP Propylparaben RS in the Standard preparation; and HU and HS are the peak heights obtained from the Test preparation and the Standard preparation, respectively.
Bacterial endotoxins  85
85 —
It contains not more than 1.0 USP Endotoxin Unit per mL.
—
It contains not more than 1.0 USP Endotoxin Unit per mL.
Particulate matter  788
788 :
meets the requirements for small-volume injections.
:
meets the requirements for small-volume injections.
Other requirements—
It responds to the Identification test and meets the requirements for pH, Iron, Heavy metals, and Assay under Sodium Chloride Injection. It meets also the requirements under Injections  1
1 .
.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Daniel K. Bempong, Ph.D.
Senior Scientist 1-301-816-8143 |
(MDPS05) Monograph Development-Pulmonary and Steroids |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
|
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance |
USP32–NF27 Page 3570
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.