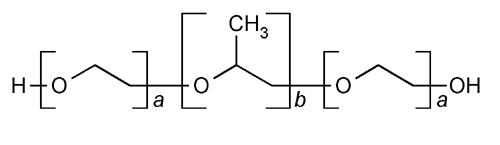
Poloxamer
HO(C2H4O)a(C3H6O)b(C2H4O)aH
Oxirane, methyl-, polymer with oxirane.
 -Hydro-
-Hydro- -hydroxypoly(oxyethylene)apoly(oxypropylene)bpoly(oxyethylene)a block copolymer, in which a and b have the following values:
-hydroxypoly(oxyethylene)apoly(oxypropylene)bpoly(oxyethylene)a block copolymer, in which a and b have the following values:
Polyethylene-polypropylene glycol

 [9003-11-6].
[9003-11-6].
Oxirane, methyl-, polymer with oxirane.
| Poloxamer | a | b |
| 124 | 12 | 20 |
| 188 | 80 | 27 |
| 237 | 64 | 37 |
| 338 | 141 | 44 |
| 407 | 101 | 56 |
Polyethylene-polypropylene glycol
» Poloxamer is a synthetic block copolymer of ethylene oxide and propylene oxide. It is available in several types, conforming to the following requirements:
| Poloxamer | Physical Form | Average Molecular Weight | Weight % Oxyethylene | Unsaturation, mEq/g |
| 124 | Liquid | 2090 to 2360 | 46.7 ± 1.9 | 0.020 ± 0.008 |
| 188 | Solid | 7680 to 9510 | 81.8 ± 1.9 | 0.026 ± 0.008 |
| 237 | Solid | 6840 to 8830 | 72.4 ± 1.9 | 0.034 ± 0.008 |
| 338 | Solid | 12700 to 17400 | 83.1 ± 1.7 | 0.031 ± 0.008 |
| 407 | Solid | 9840 to 14600 | 73.2 ± 1.7 | 0.048 ± 0.017 |
It may contain a suitable antioxidant.
Packaging and storage—
Preserve in tight containers. No storage requirements specified.
Labeling—
Label it to state, as part of the official title, the Poloxamer number. Label it to indicate the name and quantity of any antioxidant.
Identification—
Infrared Absorption  197F
197F , using a thin film of melted test specimen if it is solid. Use USP Poloxamer Liquid RS for Poloxamer 124, and use USP Poloxamer Solid RS for Poloxamer 188, 237, 338, and 407. Because of differences in the ratio of copolymer composition, the intensity of some absorption bands may vary.
, using a thin film of melted test specimen if it is solid. Use USP Poloxamer Liquid RS for Poloxamer 124, and use USP Poloxamer Solid RS for Poloxamer 188, 237, 338, and 407. Because of differences in the ratio of copolymer composition, the intensity of some absorption bands may vary.
Average molecular weight—
Phthalic anhydride–pyridine solution—
Dissolve 144 g of phthalic anhydride in freshly opened or freshly distilled pyridine containing less than 0.1% of water, and dilute with pyridine to 1000 mL. Protect from light, and allow to stand overnight. To verify that the Phthalic anhydride–pyridine solution has adequate strength, pipet 10 mL into a 250-mL conical flask, add 25 mL of pyridine and 50 mL of water, and after about 15 minutes add 0.5 mL of a solution of phenolphthalein in pyridine (1 in 100), then titrate with 0.5 N sodium hydroxide VS: it consumes between 37.6 mL and 40.0 mL of 0.5 N sodium hydroxide.
Procedure—
Accurately weigh a suitable quantity, not exceeding 15 g, of Poloxamer, calculated by multiplying the average molecular weight by 0.004, into a glass-stoppered, 250-mL boiling flask. Carefully pipet 25 mL of Phthalic anhydride–pyridine solution into the flask, touching the tip of the drained pipet to the protrusion in the flask. Add a few glass beads, and swirl to dissolve the specimen. Pipet 25 mL of Phthalic anhydride–pyridine solution into a second, glass-stoppered, conical flask, add a few glass beads, and use as the reagent blank. (An additional 25-mL portion of pyridine may be added to both the test specimen and reagent blank, prior to refluxing, if necessary to ensure fluidity.) Heat both flasks, fitted with suitable reflux condensers, and allow to reflux for 1 hour. Allow to cool, and pour two 10-mL portions of pyridine through each condenser. Remove the flasks from the condensers, add 10 mL of water to each, insert the stoppers, swirl, and allow to stand for 10 minutes. To each flask add 50.0 mL of 0.66 N sodium hydroxide and 0.5 mL of a 1 in 100 solution of phenolphthalein in pyridine. Titrate with 0.5 N sodium hydroxide VS to a light pink endpoint that persists for not less than 15 seconds, recording the volume, in mL, consumed by the residual acid in the test solution as S, and that consumed by the blank as B. Calculate the average molecular weight taken by the formula:
2000W/[(B – S)(N)]
in which W is the weight, in g, of the test specimen taken; and N is the exact normality of the 0.5 N sodium hydroxide VS.
Weight percent oxyethylene—
Solvent—
Use deuterated water or deuterochloroform.
NMR reference—
Use sodium 2,2-dimethyl-2-silapentane-5-sulfonate (for deuterated water) or tetramethylsilane (for deuterochloroform).
Test preparation—
Dissolve 0.1 g to 0.2 g of Poloxamer in deuterated water containing 1% of sodium 2,2-dimethyl-2-silapentane-5-sulfonate to obtain 1 mL of solution, or, if the Poloxamer does not dissolve in water, use deuterochloroform containing 1% of tetramethylsilane as the solvent.
Procedure—
Transfer 0.5 mL to 1.0 mL of the Test preparation to a standard 5-mm NMR spinning tube, and if deuterochloroform is the solvent, add 1 drop of deuterated water, and shake the tube. Proceed as directed for Relative Method of Quantitation under Nuclear Magnetic Resonance  761
761 , using the Test preparation volumes specified here, scanning the region from 0 ppm to 5 ppm, and using the calculation formulas specified here. Record as A1 the average area of the doublet appearing at about 1.08 ppm, representing the methyl groups of the oxypropylene units, and record as A2 the average area of the composite band from 3.2 ppm to 3.8 ppm, due to the CH2O groups of both the oxyethylene and oxypropylene units and also the CHO groups of the oxypropylene units, with reference to the sodium 2,2-dimethyl-2-silapentane-5-sulfonate or tetramethylsilane singlet at 0 ppm. Calculate the percentage of oxyethylene, by weight, in the Poloxamer, taken by the formula:
, using the Test preparation volumes specified here, scanning the region from 0 ppm to 5 ppm, and using the calculation formulas specified here. Record as A1 the average area of the doublet appearing at about 1.08 ppm, representing the methyl groups of the oxypropylene units, and record as A2 the average area of the composite band from 3.2 ppm to 3.8 ppm, due to the CH2O groups of both the oxyethylene and oxypropylene units and also the CHO groups of the oxypropylene units, with reference to the sodium 2,2-dimethyl-2-silapentane-5-sulfonate or tetramethylsilane singlet at 0 ppm. Calculate the percentage of oxyethylene, by weight, in the Poloxamer, taken by the formula:
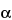 is (A2 / A1) – 1.
is (A2 / A1) – 1.
3300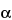 /(33
/(33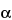 + 58)
+ 58)
in which
pH  791
791 :
between 5.0 and 7.5, in a solution (1 in 40).
:
between 5.0 and 7.5, in a solution (1 in 40).
Unsaturation—
Mercuric acetate solution—
Place 50 g of mercuric acetate in a 1000-mL volumetric flask, and dissolve with about 900 mL of methanol to which 0.5 mL of glacial acetic acid has been added. Dilute with methanol to volume, and mix. Discard the solution if it is yellow. If it is turbid, filter it. Discard it if it is still turbid. Use fresh reagents if it is necessary to repeat the preparation of the solution. Protect the solution from light by storing it in an amber bottle in the dark.
Procedure—
Transfer about 15.0 g of Poloxamer to a 250-mL conical flask. Pipet 50 mL of Mercuric acetate solution into the flask, and mix on a magnetic stirrer until solution is complete. Allow to stand for 30 minutes with occasional swirling. Add 10 g of sodium bromide crystals, and stir on a magnetic stirrer for about 2 minutes. Without delay, add about 1 mL of phenolphthalein TS, and titrate the liberated acetic acid with 0.1 N methanolic potassium hydroxide VS. Perform a blank determination. Determine also the initial acidity as follows. Dissolve 15.0 g of Poloxamer in 75 mL of methanol that has been neutralized with methanolic potassium hydroxide to the phenolphthalein endpoint. Add about 1 mL of phenolphthalein TS, and titrate with the same 0.1 N methanolic potassium hydroxide VS under a nitrogen sweep. Calculate the unsaturation, in mEq per g, taken by the formula:
(VU – VB – VA)N/15
in which VU, VB, and VA are the volumes, in mL, of 0.1 N methanolic potassium hydroxide used for titrating the test specimen, the blank, and the initial acidity, respectively; and N is the normality of the titrant.
Heavy metals, Method I  231
231 :
0.002%.
:
0.002%.
Limit of free ethylene oxide, propylene oxide, and 1,4-dioxane—
Stripped poloxamer—
Place about 500 g of Poloxamer 124 into a suitable 3-neck, round-bottom flask equipped with a stirrer, a thermometer, a vacuum outlet, and a heating mantle. Evacuate the flask carefully at room temperature to a pressure of less than 10 mm of mercury, applying the vacuum slowly to avoid excessive foaming due to entrapped gases. After any foaming has subsided, heat the flask to 80 and continue to apply vacuum for 2 hours; then cool to room temperature. Shut off the vacuum pump, and introduce nitrogen to bring the flask pressure back to atmospheric pressure. Transfer the Stripped poloxamer to a suitable nitrogen-filled container.
and continue to apply vacuum for 2 hours; then cool to room temperature. Shut off the vacuum pump, and introduce nitrogen to bring the flask pressure back to atmospheric pressure. Transfer the Stripped poloxamer to a suitable nitrogen-filled container.
Standard preparation—
[Caution—Ethylene oxide, propylene oxide, and 1,4-dioxane are toxic and flammable. Prepare these solutions in a well-ventilated fume hood.
] To a tared vial that can be sealed, add 50.0 g of Stripped poloxamer. Add 60 µL of 1,4-dioxane and 75 µL of propylene oxide from a chilled syringe. Add ethylene oxide, using the following special handling procedure. Ethylene oxide—which is a gas at room temperature—is usually stored in a lecture-type gas cylinder or a small, metal pressure-bomb. Chill the cylinder in a refrigerator before use. Transfer about 5 mL of the liquid ethylene oxide to a 100-mL beaker chilled in wet ice. Using a gas-tight syringe that has been chilled in a refrigerator, transfer 15 µL of the liquid ethylene oxide to the mixture. Immediately seal the vial, and shake on a vortex mixer for at least 30 seconds. Transfer 0.20 g of this solution to a tared vial that can be sealed, and add Stripped poloxamer to obtain a Standard preparation having a final weight of 50.0 g. Each gram of this Standard preparation contains 1 µg of ethylene oxide, 5 µg of propylene oxide, and 5 µg of 1,4-dioxane. Transfer 1.00 ± 0.01 g of this solution to a 22-mL pressure headspace vial, and add about 0.01 g of butylated hydroxytoluene. Seal with a silicone septum with or without a pressure-relief star spring and with a pressure-relief, aluminum, safety sealing-cap, and crimp the cap closed with a cap-sealing tool.
Test preparation—
Transfer 1.00 ± 0.01 g of Poloxamer to a 22-mL pressure headspace vial, and add about 0.01 g of butylated hydroxytoluene. Seal, cap, and crimp as directed for the Standard preparation.
Chromatographic system (see Chromatography  621
621 )—
The gas chromatograph is equipped with a balanced-pressure automated headspace sampler, a flame-ionization detector, and a 0.32-mm × 50-m fused-silica capillary column coated with a 5-µm layer of stationary phase G27. The column temperature is programmed from 70
)—
The gas chromatograph is equipped with a balanced-pressure automated headspace sampler, a flame-ionization detector, and a 0.32-mm × 50-m fused-silica capillary column coated with a 5-µm layer of stationary phase G27. The column temperature is programmed from 70 (10-minute initial hold) to 240
(10-minute initial hold) to 240 (10-minute final hold) at 10
(10-minute final hold) at 10 per minute, with the transfer line at 140
per minute, with the transfer line at 140 and the detector and injection port at 250
and the detector and injection port at 250 . The carrier gas is helium, flowing at a rate of about 1.6 mL per minute. Chromatograph the Standard preparation, and identify the components on the basis of the following relative retention times: about 1.0, 1.3, and 3.8, respectively, for ethylene oxide, propylene oxide, and 1,4-dioxane. Record the peak responses as directed for Procedure: the resolution, R, between ethylene oxide and propylene oxide is not less than 2.0; and the relative standard deviation for replicate injections is not more than 15%.
. The carrier gas is helium, flowing at a rate of about 1.6 mL per minute. Chromatograph the Standard preparation, and identify the components on the basis of the following relative retention times: about 1.0, 1.3, and 3.8, respectively, for ethylene oxide, propylene oxide, and 1,4-dioxane. Record the peak responses as directed for Procedure: the resolution, R, between ethylene oxide and propylene oxide is not less than 2.0; and the relative standard deviation for replicate injections is not more than 15%.
Procedure—
Separately place the vials containing the Standard preparation and the Test preparation in the automated sampler, and start the sequence so that the vial is heated at a temperature of 110 for 30 minutes before a suitable portion of its headspace is injected into the chromatograph. Set the automated sampler for a needle-withdrawal time of 0.3 minute, a pressurization time of 1 minute, an injection time of 0.08 minute, and a vial pressure of 22 psig with the vial vent off. Chromatograph the Standard preparation and the Test preparation, record the chromatograms, and measure the peak responses. Calculate the concentrations, in µg per g, of ethylene oxide, propylene oxide, and 1,4-dioxane in the portion of Poloxamer taken by the formula:
for 30 minutes before a suitable portion of its headspace is injected into the chromatograph. Set the automated sampler for a needle-withdrawal time of 0.3 minute, a pressurization time of 1 minute, an injection time of 0.08 minute, and a vial pressure of 22 psig with the vial vent off. Chromatograph the Standard preparation and the Test preparation, record the chromatograms, and measure the peak responses. Calculate the concentrations, in µg per g, of ethylene oxide, propylene oxide, and 1,4-dioxane in the portion of Poloxamer taken by the formula:
C(rU / rS)
in which C is the concentration, in µg per g, of ethylene oxide, propylene oxide, or 1,4-dioxane in the Standard preparation; and rU and rS are the peak responses obtained from the Test preparation and the Standard preparation, respectively: not more than 1 µg of ethylene oxide per g, not more than 5 µg of propylene oxide per g, and not more than 5 µg of 1,4-dioxane per g are found.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Hong Wang, Ph.D.
Scientist 1-301-816-8351 |
(EM205) Excipient Monographs 2 |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 1303
Pharmacopeial Forum: Volume No. 33(4) Page 714
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.