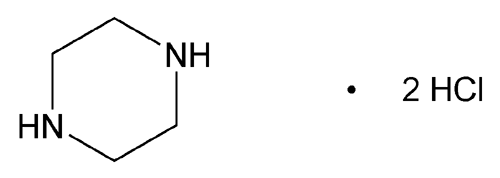
Add the following:
» Piperazine Dihydrochloride contains not less than 98.5 percent and not more than 100.5 percent of C4H10N2·2HCl, calculated on the anhydrous basis.
Packaging and storage—
Preserve in well-closed containers, and store at room temperature.
Labeling—
Label it to indicate that it is for veterinary use only.
USP Reference standards  11
11 —
USP Piperazine Dihydrochloride RS.
—
USP Piperazine Dihydrochloride RS.
Identification—
B:
In the test for Chromatographic purity, the principal spot in the chromatogram obtained from Test solution 2, observed after spraying with the ninhydrin solutions, corresponds in RF value, color, and size to that in the chromatogram obtained from Standard solution 1.
C:
It meets the requirements of the test for Chloride  191
191 .
.
pH  791
791 :
between 3.0 and 3.4, in a solution (1 in 20).
:
between 3.0 and 3.4, in a solution (1 in 20).
Water, Method I  921
921 :
not more than 10.0%.
:
not more than 10.0%.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Chromatographic purity—
Solvent—
Prepare a mixture of 13.5 N ammonium hydroxide and dehydrated alcohol (3:2).
Test solution 1—
Prepare a solution of Piperazine Dihydrochloride in Solvent containing 100 mg per mL.
Test solution 2—
Mix 1 mL of Test solution 1 and 9 mL of Solvent.
Standard solution 1—
Prepare a solution of USP Piperazine Dihydrochloride RS in Solvent containing 10 mg per mL.
Standard solution 2—
Prepare a solution of ethylenediamine in Solvent containing 0.25 mg per mL.
Standard solution 3—
Prepare a solution of triethylenediamine in Solvent containing 0.25 mg per mL.
Resolution solution—
Prepare a solution in Solvent containing 0.25 mg of triethylenediamine and 10 mg of Piperazine Dihydrochloride per mL.
Procedure—
Separately apply 5-µL portions of Test solution 1, Test solution 2, Standard solution 1, Standard solution 2, Standard solution 3, and the Resolution solution to a suitable thin-layer chromatographic plate (see Chromatography  621
621 ), coated with a 0.25-mm layer of chromatographic silica gel. Allow the spots to dry, and develop the chromatograms in a solvent system consisting of a freshly prepared mixture of acetone and 13.5 N ammonium hydroxide (80:20) until the solvent front has moved about three-fourths of the length of the plate. Remove the plates from the developing chamber, mark the solvent front, and dry the plate at 105
), coated with a 0.25-mm layer of chromatographic silica gel. Allow the spots to dry, and develop the chromatograms in a solvent system consisting of a freshly prepared mixture of acetone and 13.5 N ammonium hydroxide (80:20) until the solvent front has moved about three-fourths of the length of the plate. Remove the plates from the developing chamber, mark the solvent front, and dry the plate at 105 . Spray the plate with a 0.3% solution of ninhydrin in a mixture of butyl alcohol and glacial acetic acid (100:3). Spray the plate again with a 0.15% solution of ninhydrin in dehydrated alcohol, dry the plate at 105
. Spray the plate with a 0.3% solution of ninhydrin in a mixture of butyl alcohol and glacial acetic acid (100:3). Spray the plate again with a 0.15% solution of ninhydrin in dehydrated alcohol, dry the plate at 105 for 10 minutes, and examine the plate: any secondary spot in the chromatogram obtained from Test solution 1 is not more intense than the principal spot in the chromatogram obtained from Standard solution 2 (0.25%). Spray the plate with 0.1 N iodine TS, allow to stand for 10 minutes, and examine the plate: any spot corresponding to triethylenediamine in the chromatogram obtained from Test solution 1 is not more intense than the principal spot in the chromatogram obtained from Standard solution 3 (0.25%). In a valid test, the chromatogram obtained from the Resolution solution shows a spot due to triethylenediamine clearly separated from the principal spot. Disregard any spot at the origin of any chromatogram.
for 10 minutes, and examine the plate: any secondary spot in the chromatogram obtained from Test solution 1 is not more intense than the principal spot in the chromatogram obtained from Standard solution 2 (0.25%). Spray the plate with 0.1 N iodine TS, allow to stand for 10 minutes, and examine the plate: any spot corresponding to triethylenediamine in the chromatogram obtained from Test solution 1 is not more intense than the principal spot in the chromatogram obtained from Standard solution 3 (0.25%). In a valid test, the chromatogram obtained from the Resolution solution shows a spot due to triethylenediamine clearly separated from the principal spot. Disregard any spot at the origin of any chromatogram.
Assay—
Dissolve about 140 mg of Piperazine Dihydrochloride in 4 mL of ethylene glycol using a 150-mL beaker. Add 25 mL of glacial acetic acid containing 1.2 g of mercuric acetate, rinsing the walls of the beaker with a small amount of the glacial acetic acid. Add 0.25 mL of p-naphtholbenzein TS, and titrate with 0.1 N perchloric acid VS. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 7.953 mg of C4H10N2 · 2HCl. USP32
USP32
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Ian DeVeau, Ph.D.
Director, Veterinary Drugs and Radiopharmaceuticals 1-301-816-8178 |
(VET05) Veterinary Drugs 05 |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 3315
Pharmacopeial Forum: Volume No. 33(6) Page 1202