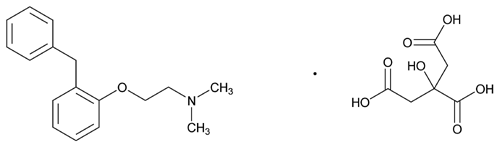
Phenyltoloxamine Citrate
» Phenyltoloxamine Citrate contains not less than 99.0 percent and not more than 101.0 percent of C17H21NO·C6H8O7, calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers. Store at room temperature.
USP Reference standards  11
11 —
—
USP Phenyltoloxamine Citrate RS.
USP Phenyltoloxamine Related Compound A RS.
USP Phenyltoloxamine Citrate RS.
USP Phenyltoloxamine Related Compound A RS.
Identification,
Infrared Absorption  197K
197K .
.
pH  791
791 :
between 3.2 and 4.2, in a solution (1 in 100).
:
between 3.2 and 4.2, in a solution (1 in 100).
Loss on drying  731
731 —
Dry it in vacuum at 80
—
Dry it in vacuum at 80 for 3 hours: it loses not more than 0.5% of its weight.
for 3 hours: it loses not more than 0.5% of its weight.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Heavy metals, Method I  231
231 :
20 µg per g.
:
20 µg per g.
Related compounds—
Resolution solution—
In a separatory funnel dissolve about 10 mg each of USP Phenyltoloxamine Citrate RS and USP Phenyltoloxamine Related Compound A RS, accurately weighed, in 50 mL of water. Add 5 mL of ammonium hydroxide, and extract with three 10-mL portions of methylene chloride. Combine the extracts, dry the solution over anhydrous sodium sulfate, and gently evaporate to dryness. Dissolve the residue in 20 mL of methylene chloride.
Test solution—
In a separatory funnel dissolve about 400 mg of Phenyltoloxamine Citrate, accurately weighed, in 50 mL of water. Proceed as directed for Resolution solution, beginning with “Add 5 mL of ammonium hydroxide.”
Chromatographic system (see Chromatography  621
621 )—
The gas chromatograph is equipped with a split injection system, a flame-ionization detector, and a 0.32-mm × 25-m column coated with a 0.45-µm film of phase G27. The carrier gas is helium, flowing at a rate of about 29 cm per second, with a split flow rate of about 25 mL per minute. The column temperature is programmed as follows. Initially the temperature of the column is equilibrated at 190
)—
The gas chromatograph is equipped with a split injection system, a flame-ionization detector, and a 0.32-mm × 25-m column coated with a 0.45-µm film of phase G27. The carrier gas is helium, flowing at a rate of about 29 cm per second, with a split flow rate of about 25 mL per minute. The column temperature is programmed as follows. Initially the temperature of the column is equilibrated at 190 for 3 minutes, then the temperature is increased at a rate of 4
for 3 minutes, then the temperature is increased at a rate of 4 per minute to 240
per minute to 240 , and maintained at 240
, and maintained at 240 for 8 minutes. The injection port and the detector temperatures are maintained at 280
for 8 minutes. The injection port and the detector temperatures are maintained at 280 . Chromatograph the Resolution solution, and record the peak responses as directed for Procedure: the resolution, R, between phenyltoloxamine and phenyltoloxamine related compound A is not less than 1.5.
. Chromatograph the Resolution solution, and record the peak responses as directed for Procedure: the resolution, R, between phenyltoloxamine and phenyltoloxamine related compound A is not less than 1.5.
Procedure—
Inject a volume (about 1 µL) of the Test solution into the chromatograph, record the chromatograms, and measure the peak responses. Calculate the percentage of each impurity in the portion of Phenyltoloxamine Citrate taken by the formula:
100(ri / rs)
in which ri is the peak response of each impurity; and rs is the sum of the responses of all the peaks, excluding the solvent peaks: not more than 0.2% of phenyltoloxamine related compound A; not more than 0.1% of any other individual impurity; and not more than 1.0% of total impurities is found.
Assay—
Dissolve about 0.5 g of Phenyltoloxamine Citrate, accurately weighed, in 80 mL of glacial acetic acid, and titrate with 0.1 N perchloric acid VS, determining the endpoint potentiometrically. Perform a blank determination, and make any necessary correction (see Titrimetry  541
541 ). Each mL of 0.1 N perchloric acid is equivalent to 44.75 mg of C17H21NO·C6H8O7.
). Each mL of 0.1 N perchloric acid is equivalent to 44.75 mg of C17H21NO·C6H8O7.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Daniel K. Bempong, Ph.D.
Senior Scientist 1-301-816-8143 |
(MDPS05) Monograph Development-Pulmonary and Steroids |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 3291
Pharmacopeial Forum: Volume No. 30(4) Page 1291