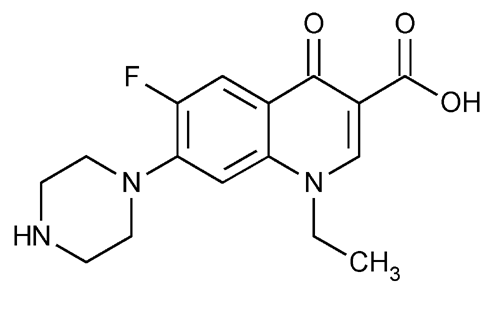
Norfloxacin
3-Quinolinecarboxylic acid, 1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-.
1-Ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid
» Norfloxacin contains not less than 99.0 percent and not more than 101.0 percent of C16H18FN3O3, calculated on the dried basis.
Packaging and storage—
Preserve in tight, light-resistant containers.
Identification—
B:
Ultraviolet Absorption  197U
197U —[Note—Use-low actinic glassware in this procedure.]
—[Note—Use-low actinic glassware in this procedure.]
Solution:
5 µg per mL.
Medium:
0.1 N sodium hydroxide.
Absorptivities at 273 nm, calculated on the dried basis, do not differ by more than 3.0%.
Loss on drying  731
731 —
Dry it in vacuum at a pressure not exceeding 5 mm of mercury at 100
—
Dry it in vacuum at a pressure not exceeding 5 mm of mercury at 100 to constant weight: it loses not more than 1.0% of its weight.
to constant weight: it loses not more than 1.0% of its weight.
Residue on ignition  281
281 :
not more than 0.1%, a platinum crucible being used.
:
not more than 0.1%, a platinum crucible being used.
Heavy metals, Method II  231
231 :
0.0015%.
:
0.0015%.
Chromatographic purity—
Dissolve a quantity of Norfloxacin in a mixture of methanol and methylene chloride (1:1) to obtain a test solution containing 8.0 mg per mL. Dissolve 4.0 mg of USP Norfloxacin RS in 1 mL of glacial acetic acid, add 4 mL of methanol, and mix. To 1 mL of this Standard stock solution add 9 mL of the mixture of methanol and methylene chloride (1:1) to obtain Comparison solution A. Dilute a portion of this solution with an equal volume of the mixture of methanol and methylene chloride (1:1) to obtain Comparison solution B. Separately apply 5 µL of the test solution, 1, 1.5, and 2 µL of Comparison solution A, and 5 µL of Comparison solution B to a suitable high-performance thin-layer chromatographic plate (see Chromatography  621
621 ) coated with a 0.25-mm layer of silica gel mixture, previously washed with methanol and air-dried. The spots of Comparison solutions A and B are equivalent to 0.2, 0.3, 0.4, and 0.5% of impurities, respectively. Place the plate in a paper-lined chromatographic chamber previously equilibrated with a solvent system consisting of a mixture of chloroform, methanol, toluene, diethylamine, and water (40:40:20:14:8). Seal the chamber and allow the chromatogram to develop until the solvent front has moved about nine-tenths of the length of the plate. Remove the plate from the chamber, mark the solvent front, allow the solvent to evaporate, and examine the plate under both short- and long-wavelength UV light. Compare the intensities of any secondary spots observed in the chromatogram of the test solution with those of the principal spots in the chromatograms of Comparison solutions A and B: the sum of the intensities of secondary spots obtained from the test solution corresponds to not more than 0.5% of impurities.
) coated with a 0.25-mm layer of silica gel mixture, previously washed with methanol and air-dried. The spots of Comparison solutions A and B are equivalent to 0.2, 0.3, 0.4, and 0.5% of impurities, respectively. Place the plate in a paper-lined chromatographic chamber previously equilibrated with a solvent system consisting of a mixture of chloroform, methanol, toluene, diethylamine, and water (40:40:20:14:8). Seal the chamber and allow the chromatogram to develop until the solvent front has moved about nine-tenths of the length of the plate. Remove the plate from the chamber, mark the solvent front, allow the solvent to evaporate, and examine the plate under both short- and long-wavelength UV light. Compare the intensities of any secondary spots observed in the chromatogram of the test solution with those of the principal spots in the chromatograms of Comparison solutions A and B: the sum of the intensities of secondary spots obtained from the test solution corresponds to not more than 0.5% of impurities.
Assay—
Dissolve about 460 mg of Norfloxacin, accurately weighed, in 100 mL of glacial acetic acid. Titrate potentiometrically with 0.1 N perchloric acid VS using a suitable anhydrous electrode system (see Titrimetry  541
541 ). [note—Remove any aqueous solution in the electrode(s), render anhydrous, and fill with 0.1 N lithium perchlorate in acetic anhydride.] Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 31.93 mg of C16H18FN3O3.
). [note—Remove any aqueous solution in the electrode(s), render anhydrous, and fill with 0.1 N lithium perchlorate in acetic anhydride.] Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 31.93 mg of C16H18FN3O3.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Behnam Davani, Ph.D., M.B.A.
Senior Scientist 1-301-816-8394 |
(MDAA05) Monograph Development-Antivirals and Antimicrobials |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 3111