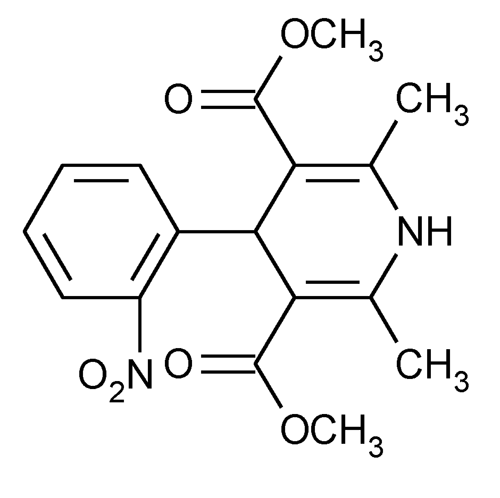
Nifedipine
» Nifedipine contains not less than 98.0 percent and not more than 102.0 percent of C17H18N2O6, calculated on the dried basis.
Packaging and storage—
Preserve in tight, light-resistant containers.
USP Reference standards  11
11 —
—
USP Nifedipine RS.
USP Nifedipine Nitrophenylpyridine Analog RS.
USP Nifedipine Nitrosophenylpyridine Analog RS.
USP Nifedipine RS.
USP Nifedipine Nitrophenylpyridine Analog RS.
USP Nifedipine Nitrosophenylpyridine Analog RS.
note—Nifedipine, when exposed to daylight and certain wavelengths of artificial light, readily converts to a nitrosophenylpyridine derivative. Exposure to UV light leads to the formation of a nitrophenylpyridine derivative. Perform assays and tests in the dark or under golden fluorescent or other low-actinic light. Use low-actinic glassware.
Identification—
A:
Infrared Absorption  197K
197K —Do not dry specimens.
—Do not dry specimens.
B: Ultraviolet Absorption
Ultraviolet Absorption  197U
197U —
—
Spectral range:
450 to 200 nm.
Solution—
To a 10-mL volumetric flask containing 14 mg of Nifedipine add 1.0 mL of chloroform, dilute with methanol to volume, and mix. Pipet a 1.0-mL aliquot of the solution into a 100-mL volumetric flask, dilute with methanol to volume, and mix.
Medium:
methanol.
C:
The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay.
Loss on drying  731
731 —
Dry it at 105
—
Dry it at 105 to constant weight: it loses not more than 0.5% of its weight.
to constant weight: it loses not more than 0.5% of its weight.
Residue on ignition  281
281 :
not more than 0.1%, an ignition temperature of 600
:
not more than 0.1%, an ignition temperature of 600 being used.
being used.
Heavy metals, Method II  231
231 :
0.001%.
:
0.001%.
Perchloric acid titration—
Transfer about 4 g of Nifedipine, accurately weighed, to a 250-mL conical flask, and dissolve in 160 mL of glacial acetic acid with the aid of an ultrasonic bath. Add 3 drops of p-naphtholbenzein TS, and titrate to a green endpoint with 0.1 N perchloric acid VS: not more than 0.12 mL of 0.1 N perchloric acid is consumed for each g of Nifedipine.
Chloride and Sulfate—
To 5.0 g of Nifedipine in a 140-mL beaker add 4.0 mL of 6 N acetic acid and 46 mL of water. Bring carefully to a boil on a hot plate, cool, and filter through paper free of chloride and sulfate. Use this Nifedipine filtrate for the following tests.
Chloride—
Pipet 2.5 mL of the Nifedipine filtrate into a 50-mL color-comparison tube, and add 12.5 mL of water. Into a matched color-comparison tube pipet 10 mL of a Standard solution containing 8.2 µg of sodium chloride per mL corresponding to 5 µg of chloride per mL, add 5.0 mL of water, and mix. To each tube add 0.15 mL of 0.3 M nitric acid and 0.3 mL of silver nitrate TS, and mix: the opalescence exhibited by the Nifedipine filtrate does not exceed that of the Standard solution (0.02%).
Sulfate—
Pipet into each of two 50-mL matched color-comparison tubes 1.5 mL of sulfate solution consisting of sufficient potassium sulfate dissolved in water to obtain a sulfate concentration of 10 µg per mL. To each tube add, successively and with continuous shaking, 0.75 mL of alcohol, 0.5 mL of a 6.1% aqueous solution of barium chloride, and 0.25 mL of 6 N acetic acid. Shake for an additional 30 seconds. Pipet into one tube, designated the Standard tube, 15 mL of the sulfate solution. Pipet into the other tube, designated the Specimen tube, 3 mL of the Nifedipine filtrate and 12 mL of water: the turbidity exhibited by the Specimen tube does not exceed that of the Standard tube (0.05%).
Related compounds—
[note—Protect the Standard nifedipine solution and the Test preparation from actinic light. Conduct this test promptly after preparation of the Standard nifedipine solution and the Test solution.]
Mobile phase—
Prepare as directed in the Assay.
Standard nifedipine solution—
Dissolve an accurately weighed quantity of USP Nifedipine RS in methanol (about 1 mg per mL), and dilute quantitatively with Mobile phase to obtain a solution having a known concentration of about 0.3 mg per mL.
Reference solution 1—
Dissolve an accurately weighed quantity of USP Nifedipine Nitrophenylpyridine Analog RS in methanol (about 1 mg per mL), and dilute quantitatively with Mobile phase to obtain a solution having a known concentration of about 0.6 µg per mL.
Reference solution 2—
Dissolve an accurately weighed quantity of USP Nifedipine Nitrosophenylpyridine Analog RS in methanol (about 1 mg per mL), and dilute quantitatively with Mobile phase to obtain a solution having a known concentration of about 0.6 µg per mL.
Standard solution—
Transfer 5.0 mL of each of the two Reference solutions to a container, add 5.0 mL of Mobile phase, and mix.
Test solution—
Prepare as directed for the Assay preparation in the Assay.
System suitability solution—
Mix equal volumes of the Standard nifedipine solution and of each of the two Reference solutions.
Chromatographic system—
Prepare as directed in the Assay. Chromatograph the System suitability preparation, and record the peak responses as directed for Procedure: the resolution, R, between the nitrophenylpyridine analog and nitrosophenylpyridine analog peaks is not less than 1.5; the resolution, R, between the nitrosophenylpyridine analog and nifedipine peaks is not less than 1.0; and the relative standard deviation of the response for each analog in replicate injections is not more than 10%. The relative retention times are about 0.8 for the nitrophenylpyridine analog, about 0.9 for the nitrosophenylpyridine analog, and 1.0 for nifedipine.
Procedure—
Separately inject equal volumes (about 25 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of each related compound in the portion of Nifedipine taken by the formula:
250C(rU / rS)
in which C is the concentration, in mg per mL, of the appropriate USP Nifedipine Analog RS, in the Standard solution; and rU and rS are the peak responses for the corresponding related compound obtained from the Test solution and the Standard solution, respectively. Not more than 0.2% of each of dimethyl 4-(2-nitrophenyl)-2,6-dimethylpyridine-3,5-dicarboxylate and dimethyl- 4-(2-nitrosophenyl)-2,6-dimethylpyridine-3,5-dicarboxylate, corresponding to Nifedipine Nitrophenylpyridine Analog and Nifedipine Nitrosophenylpyridine Analog, respectively, is found.
Assay—
[note—Protect the Standard preparation and the Assay preparation from actinic light. Conduct the Assay promptly after preparation of the Standard preparation and the Assay preparation.]
Mobile phase—
Prepare a suitable mixture of water, acetonitrile, and methanol (50:25:25), and degas. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard preparation—
Dissolve an accurately weighed quantity of USP Nifedipine RS in methanol (about 1 mg per mL), and quantitatively dilute with Mobile phase to obtain a solution having a known concentration of about 0.1 mg per mL.
Assay preparation—
Transfer about 25 mg of Nifedipine, accurately weighed, to a 250-mL volumetric flask. Dissolve in 25 mL of methanol, dilute with Mobile phase to volume, and mix to obtain a solution having a concentration of about 0.1 mg per mL.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 235-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L1. The flow rate is about 1.0 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the column efficiency is not less than 4000 theoretical plates; the tailing factor is not more than 1.5; and the relative standard deviation for replicate injections is not more than 1.0%.
)—
The liquid chromatograph is equipped with a 235-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L1. The flow rate is about 1.0 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the column efficiency is not less than 4000 theoretical plates; the tailing factor is not more than 1.5; and the relative standard deviation for replicate injections is not more than 1.0%.
Procedure—
Separately inject equal volumes (about 25 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of C17H18N2O6 in the portion of Nifedipine taken by the formula:
250C(rU / rS)
in which C is the concentration, in mg per mL, of USP Nifedipine RS in the Standard preparation; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Sujatha Ramakrishna, Ph.D.
Scientist 1-301-816-8349 |
(MDCV05) Monograph Development-Cardiovascular |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 3083
Pharmacopeial Forum: Volume No. 27(3) Page 2569
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.