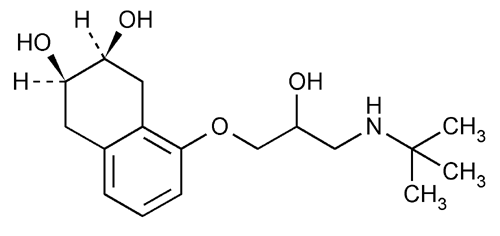
Nadolol
2,3-Naphthalenediol, 5-[3-[(1,1-dimethylethyl)amino]-2-hydroxypropoxy]-1,2,3,4-tetrahydro-, cis-.
1-(tert-Butylamino)-3-[(5,6,7,8-tetrahydro-cis-6,7-dihydroxy-1-naphthyl)oxy]-2-propanol
» Nadolol contains not less than 98.0 percent and not more than 101.5 percent of C17H27NO4, calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers.
Identification, Infrared Absorption  197M
197M .
.
Loss on drying  731
731 —
Dry it in vacuum at 60
—
Dry it in vacuum at 60 for 3 hours: it loses not more than 2.0% of its weight.
for 3 hours: it loses not more than 2.0% of its weight.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Heavy metals, Method II  231
231 :
0.003%.
:
0.003%.
Racemate composition—
Prepare a mineral oil dispersion of Nadolol, previously dried, adjusting the thickness of the mull to give an absorbance reading of 0.6 ± 0.1 at 6.3 µm. Record the spectrum from 6 to 9 µm, using mineral oil in the reference beam. Calculate the percentage of racemate A in the portion of Nadolol taken by the formula:
(50 / 0.9)(Aa / Ab)
in which 0.9 is the average value of (Aa/Ab) in a mixture of racemates A and B (1:1), Aa is the uncorrected absorbance at the wavelength of a maximum absorbance at about 7.90 µm, corresponding to racemate A, and Ab is the uncorrected absorbance at the wavelength of a maximum absorbance at about 8.00 µm, corresponding to racemate B: the content of racemate A is between 40% and 60%.
Chromatographic purity—
Prepare the test solution by dissolving about 500 mg of Nadolol in 10.0 mL of a mixture of methanol and chloroform (1:1). Prepare a solution of USP Nadolol RS in a mixture of methanol and chloroform (1:1) containing about 50 mg per mL. [note—This Standard solution is used only to identify the nadolol zone.] Divide a thin-layer chromatographic plate (see Chromatography  621
621 ) coated with a 0.25-mm layer of chromatographic silica gel mixture, into four equal sections, the first section to be used for the Standard solution, the next two sections to be used for the test solution, and the last section for the blank. Apply, as streaks, 100 µL of the Standard solution, two 100-µL portions of the test solution, and 100 µL of the mixture of methanol and chloroform (1:1) to provide the blank to appropriate sections of the plate, drying each solution as it is applied with a current of cool air. Develop the chromatogram in a solvent system consisting of a mixture of acetone, chloroform, and 2 M ammonium hydroxide (8:1:1) until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the developing chamber, air-dry, and locate the bands by viewing under short-wavelength UV light. Identify the nadolol zones by comparison of the chromatograms of the test solution with the chromatogram of the Standard solution. Mark the nadolol zones and the separated impurity zones in the chromatograms of the test solution, and the corresponding zones in the blank section of the plate. Remove the silica gel from the nadolol zone in each chromatogram of the test solution, and transfer to separate 50-mL centrifuge tubes, and similarly transfer the silica gel removed from the corresponding area of the blank chromatogram to a third 50-mL centrifuge tube. Remove the silica gel from the combined impurity zones in each chromatogram of the test solution, and transfer to separate 50-mL centrifuge tubes, and similarly transfer the silica gel removed from the corresponding area of the blank chromatogram to a sixth 50-mL centrifuge tube. Add 30.0 mL of alcohol to each of the 2 tubes containing the mixtures from the nadolol zones and to the third tube containing the corresponding portion of the blank mixture, and add 10.0 mL of alcohol to each of the 2 tubes containing the mixtures from the impurity zones and to the sixth tube containing the corresponding portion of the blank mixture. Shake for 60 minutes, and centrifuge to clarify. Concomitantly determine the absorbances of the supernatants at the wavelength of maximum absorbance at about 278 nm, with a suitable spectrophotometer, using alcohol as the spectrophotometer blank. Calculate the percentage of impurities in the portion of Nadolol taken by the formula:
) coated with a 0.25-mm layer of chromatographic silica gel mixture, into four equal sections, the first section to be used for the Standard solution, the next two sections to be used for the test solution, and the last section for the blank. Apply, as streaks, 100 µL of the Standard solution, two 100-µL portions of the test solution, and 100 µL of the mixture of methanol and chloroform (1:1) to provide the blank to appropriate sections of the plate, drying each solution as it is applied with a current of cool air. Develop the chromatogram in a solvent system consisting of a mixture of acetone, chloroform, and 2 M ammonium hydroxide (8:1:1) until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the developing chamber, air-dry, and locate the bands by viewing under short-wavelength UV light. Identify the nadolol zones by comparison of the chromatograms of the test solution with the chromatogram of the Standard solution. Mark the nadolol zones and the separated impurity zones in the chromatograms of the test solution, and the corresponding zones in the blank section of the plate. Remove the silica gel from the nadolol zone in each chromatogram of the test solution, and transfer to separate 50-mL centrifuge tubes, and similarly transfer the silica gel removed from the corresponding area of the blank chromatogram to a third 50-mL centrifuge tube. Remove the silica gel from the combined impurity zones in each chromatogram of the test solution, and transfer to separate 50-mL centrifuge tubes, and similarly transfer the silica gel removed from the corresponding area of the blank chromatogram to a sixth 50-mL centrifuge tube. Add 30.0 mL of alcohol to each of the 2 tubes containing the mixtures from the nadolol zones and to the third tube containing the corresponding portion of the blank mixture, and add 10.0 mL of alcohol to each of the 2 tubes containing the mixtures from the impurity zones and to the sixth tube containing the corresponding portion of the blank mixture. Shake for 60 minutes, and centrifuge to clarify. Concomitantly determine the absorbances of the supernatants at the wavelength of maximum absorbance at about 278 nm, with a suitable spectrophotometer, using alcohol as the spectrophotometer blank. Calculate the percentage of impurities in the portion of Nadolol taken by the formula:
100Ai / (Ai + 3AU)
in which Ai is the average absorbance of the impurity zone eluates corrected for the corresponding blank, and AU is the average absorbance of the nadolol zone eluates corrected for the corresponding blank: not more than 2.0% is found.
Assay—
Perchloric acid titrant—
Mix 8.5 mL of perchloric acid with 500 mL of glacial acetic acid, cool, dilute with glacial acetic acid to 1000 mL, and mix. Standardize this solution as directed for Perchloric Acid, Tenth-Normal (0.1 N) (in Glacial Acetic Acid) in the section Volumetric Solutions under Reagents, Indicators, and Solutions.
Procedure—
Transfer about 280 mg of Nadolol, accurately weighed, to a 250-mL conical flask. Add 100 mL of glacial acetic acid, and place in an ultrasonic bath until solution is complete. Add 2 drops of crystal violet TS, and titrate with Perchloric acid titrant to an emerald-green endpoint. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 30.94 mg of C17H27NO4.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Sujatha Ramakrishna, Ph.D.
Scientist 1-301-816-8349 |
(MDCV05) Monograph Development-Cardiovascular |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 3019
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.