Mono- and Di-glycerides
» Mono- and Di-glycerides is a mixture of glycerol mono- and di-esters, with minor amounts of tri-esters, of fatty acids from edible oils. It contains not less than 40.0 percent of monoglycerides. The monoglyceride content is not less than 90.0 percent and not more than 110.0 percent of the value indicated in the labeling. It may contain suitable stabilizers.
Packaging and storage—
Preserve in tight, light-resistant containers.
Labeling—
The labeling indicates the monoglyceride content, hydroxyl value, iodine value, saponification value, and name and quantity of any stabilizers.
USP Reference standards  11
11 —
—
USP Glycerin RS.
USP Glycerin RS.
Acid value  401
401 :
not more than 4.
:
not more than 4.
Hydroxyl value  401
401 :
not less than 90.0% and not more than 110.0% of the value indicated in the labeling.
:
not less than 90.0% and not more than 110.0% of the value indicated in the labeling.
Iodine value  401
401 :
not less than 90.0% and not more than 110.0% of the value indicated in the labeling. If the value stated in the labeling is less than 10, the Iodine value is not more than 10.
:
not less than 90.0% and not more than 110.0% of the value indicated in the labeling. If the value stated in the labeling is less than 10, the Iodine value is not more than 10.
Saponification value  401
401 :
not less than 90.0% and not more than 110.0% of the value indicated in the labeling.
:
not less than 90.0% and not more than 110.0% of the value indicated in the labeling.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Arsenic, Method II  211
211 :
3 ppm.
:
3 ppm.
Heavy metals, Method II  231
231 :
0.001%.
:
0.001%.
Limit of free glycerin—
Mobile phase and Chromatographic system—
Proceed as described in the Assay for monoglycerides.
Standard solutions—
Dissolve an accurately weighed quantity of USP Glycerin RS in tetrahydrofuran, and dilute with tetrahydrofuran, as necessary, to obtain solutions having known concentrations of about 0.5 mg per mL, 1.0 mg per mL 2.0 mg per mL, and 4.0 mg per mL.
Test solution—
Use the Assay preparation.
Procedure—
Separately inject equal volumes (about 40 µL) of each of the Standard solutions and the Test solution into the chromatograph, record the chromatograms, and measure the responses for the glycerin peaks. Plot the concentration, in mg per mL, of USP Glycerin RS in the Standard solutions versus the glycerin peak responses obtained. From the standard curve so obtained, determine the glycerin concentration, C, in mg per mL, in the Test solution. Calculate the percentage of glycerin in the portion of Mono- and Di-glycerides taken by the formula:
500(C/W)
in which C is as obtained above; and W is the amount, in mg, of Mono- and Di-glycerides taken to prepare the Test solution: not more than 7.0% of free glycerin is found.
Assay for monoglycerides—
Mobile phase—
Use filtered and degassed tetrahydrofuran. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Assay preparation—
Transfer about 200 mg of Mono- and Di-glycerides, accurately weighed, to a 5-mL volumetric flask, dissolve in and dilute with tetrahydrofuran to volume, and mix.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a refractive index detector and a 7-mm × 60-cm column that contains 5-µm packing L21 (100
)—
The liquid chromatograph is equipped with a refractive index detector and a 7-mm × 60-cm column that contains 5-µm packing L21 (100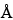 ). The flow rate is about 1 mL per minute. The column and detector temperatures are maintained at 40
). The flow rate is about 1 mL per minute. The column and detector temperatures are maintained at 40 . [note—Two or three 7.5-mm × 30-cm L21 columns may be used in place of one 60-cm column provided that system suitability requirements are met.] Chromatograph the Assay preparation, and record the peak responses as directed for Procedure. The order of elution is triglycerides, diglycerides, monoglycerides, and glycerin. The relative standard deviation for replicate injections determined from the monoglycerides peak is not more than 1.0%.
. [note—Two or three 7.5-mm × 30-cm L21 columns may be used in place of one 60-cm column provided that system suitability requirements are met.] Chromatograph the Assay preparation, and record the peak responses as directed for Procedure. The order of elution is triglycerides, diglycerides, monoglycerides, and glycerin. The relative standard deviation for replicate injections determined from the monoglycerides peak is not more than 1.0%.
Procedure—
Inject a volume (about 40 µL) of the Assay preparation into the chromatograph, record the chromatogram, and measure the responses for the major peaks. Calculate the percentage of monoglycerides in the portion of Mono- and Di-glycerides taken by the formula:
100(rU / rS)
in which rU is the peak response for monoglycerides; and rS is the sum of the responses of all the peaks, except the solvent peak.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Robert H. Lafaver, B.A.
Scientist 1-301-816-8335 |
(EM105) Excipient Monographs 1 |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 1283
Pharmacopeial Forum: Volume No. 30(4) Page 1330
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.