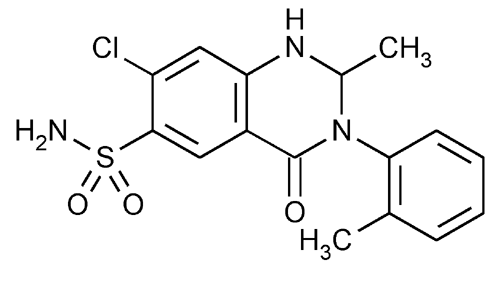
Metolazone
6-Quinazolinesulfonamide, 7-chloro-1,2,3,4-tetrahydro-2-methy1-3-(2-methylphenyl)-4-oxo-.
7-Chloro-1,2,3,4-tetrahydro-2-methyl-4-oxo-3-o-tolyl-6-quinazolinesulfonamide
» Metolazone contains not less than 97.0 percent and not more than 102.0 percent of C16H16ClN3O3S, calculated on the dried basis.
Packaging and storage—
Preserve in tight, light-resistant containers.
Identification—
Loss on drying  731
731 —
Dry it at 105
—
Dry it at 105 for 2 hours: it loses not more than 1.0% of its weight.
for 2 hours: it loses not more than 1.0% of its weight.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Heavy metals, Method II  231
231 :
0.0015%.
:
0.0015%.
Chromatographic purity—
[note—Protect Metolazone solutions from light.]
Standard preparations—
Dissolve an accurately weighed quantity of USP Metolazone RS in tetrahydrofuran and mix to obtain Standard preparation A having a known concentration of 0.50 mg per mL. Dilute a portion of Standard preparation A quantitatively with tetrahydrofuran to obtain Standard preparation B having a known concentration of 0.25 mg per mL.
Test preparation—
Dissolve an accurately weighed quantity of Metolazone in tetrahydrofuran to obtain a solution containing 50 mg per mL.
Procedure—
Separately apply 10 µL of the Test preparation and each of the two Standard preparations to a suitable thin-layer chromatographic plate (see Chromatography  621
621 ) coated with a 0.25-mm layer of chromatographic silica gel mixture. Allow the spots to dry, and develop the chromatogram in a solvent system consisting of a mixture of chloroform, ethyl acetate, and formic acid (55:40:5) until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the developing chamber, mark the solvent front, air-dry, examine the plate under short-wavelength UV light, and compare the intensities of any secondary spots observed in the chromatogram of the Test preparation with those of the principal spots in the chromatograms of the Standard preparations: no secondary spot from the chromatogram of the Test preparation is larger or more intense than the principal spot obtained from Standard preparation B (0.5%) and the sum of the intensities of the secondary spots obtained from the Test preparation corresponds to not more than 1.0%.
) coated with a 0.25-mm layer of chromatographic silica gel mixture. Allow the spots to dry, and develop the chromatogram in a solvent system consisting of a mixture of chloroform, ethyl acetate, and formic acid (55:40:5) until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the developing chamber, mark the solvent front, air-dry, examine the plate under short-wavelength UV light, and compare the intensities of any secondary spots observed in the chromatogram of the Test preparation with those of the principal spots in the chromatograms of the Standard preparations: no secondary spot from the chromatogram of the Test preparation is larger or more intense than the principal spot obtained from Standard preparation B (0.5%) and the sum of the intensities of the secondary spots obtained from the Test preparation corresponds to not more than 1.0%.
Assay—
[note—Use low-actinic glassware throughout the Assay.]
Standard preparation—
Dissolve an accurately weighed quantity of USP Metolazone RS in methanol to obtain a solution having a known concentration of about 40 µg per mL.
Assay preparation—
Transfer about 50 mg of Metolazone, accurately weighed, to 100-mL volumetric flask, dilute with methanol to volume, and mix. Pipet 20 mL into 250-mL volumetric flask, dilute with methanol to volume, and mix.
Procedure—
Concomitantly determine the absorbances of the solutions at the wavelength of maximum absorbance at about 343 nm, with a suitable spectrophotometer, using methanol as the blank. Calculate the quantity, in mg, of C16H16ClN3O3S in the portion of Metolazone taken by the formula:
1.25C(AU / AS)
in which C is the concentration, in µg per mL, of USP Metolazone RS in the Standard preparation; and AU and AS are the absorbances of the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Sujatha Ramakrishna, Ph.D.
Scientist 1-301-816-8349 |
(MDCV05) Monograph Development-Cardiovascular |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 2961
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.