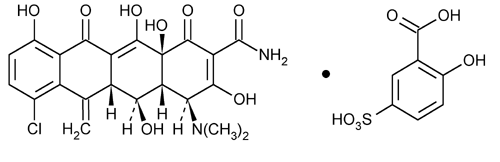
Meclocycline Sulfosalicylate
2-Naphthacenecarboxamide, 7-chloro-4-(dimethylamino)- 1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methylene-1,11-dioxo-, [4S-(4
(4S,4aR,5S,5aR,12aS)-7-Chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methylene-1,11-dioxo-2-naphthacene carboxamide mono(5-sulfosalicylate) (salt)
» Meclocycline Sulfosalicylate has a potency equivalent to not less than 620 µg of meclocycline (C22H21ClN2O8) per mg.
Packaging and storage—
Preserve in tight containers, protected from light.
Identification, Infrared Absorption  197K
197K .
.
Crystallinity  695
695 :
meets the requirements.
:
meets the requirements.
pH  791
791 :
between 2.5 and 3.5, in a solution containing 10 mg per mL.
:
between 2.5 and 3.5, in a solution containing 10 mg per mL.
Water, Method I  921
921 :
not more than 4.0%.
:
not more than 4.0%.
Assay—
0.001 M Ammonium edetate—
Transfer 293 mg of edetic acid, accurately weighed, to a 1000-mL volumetric flask, add 1 mL of methanol and 7 mL of ammonium hydroxide, and shake to dissolve the edetic acid. Add 900 mL of water, adjust with glacial acetic acid to a pH of 6.6, dilute with water to volume, and mix.
Mobile phase—
Prepare a solution of 0.001 M Ammonium edetate and tetrahydrofuran (85:15). Filter and degas the solution before use.
Standard preparation—
Transfer 36 mg of USP Meclocycline Sulfosalicylate RS, accurately weighed, to a 50-mL volumetric flask, dilute with methanol to volume, and mix. Transfer 3.0 mL of this solution to a 25-mL volumetric flask, dilute with Mobile phase to volume, and mix to obtain a solution having a known concentration of about 60 µg of meclocycline per mL.
Assay preparation—
Using 36 mg of Meclocycline Sulfosalicylate, accurately weighed, prepare as directed for Standard preparation.
Procedure—
Introduce equal volumes (about 10 µL) of the Assay preparation and the Standard preparation into a high-pressure liquid chromatograph (see Chromatography  621
621 ), operated at room temperature, by means of a suitable microsyringe or sampling valve, adjusting the specimen size and other operating parameters such that the peak obtained from the Standard preparation is about 0.6 full-scale. Typically, the apparatus is fitted with a 25-cm × 4-mm column packed with packing L1 and is equipped with an UV detector capable of monitoring absorption at 340 nm, and a suitable recorder. In a suitable chromatogram the coefficient of variation for replicate injections of the Standard preparation is not more than 3.0%. Measure the peak responses at equivalent retention times, obtained from the Assay preparation and the Standard preparation, and calculate the quantity, in mg, of C22H21ClN2O8 in the portion of Meclocycline Sulfosalicylate taken by the formula:
), operated at room temperature, by means of a suitable microsyringe or sampling valve, adjusting the specimen size and other operating parameters such that the peak obtained from the Standard preparation is about 0.6 full-scale. Typically, the apparatus is fitted with a 25-cm × 4-mm column packed with packing L1 and is equipped with an UV detector capable of monitoring absorption at 340 nm, and a suitable recorder. In a suitable chromatogram the coefficient of variation for replicate injections of the Standard preparation is not more than 3.0%. Measure the peak responses at equivalent retention times, obtained from the Assay preparation and the Standard preparation, and calculate the quantity, in mg, of C22H21ClN2O8 in the portion of Meclocycline Sulfosalicylate taken by the formula:
(1.25 / 3)(C)(rU / rS)
in which C is the equivalent, in µg per mL, of meclocycline from the USP Meclocycline Sulfosalicylate RS in the Standard preparation; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Ahalya Wise, M.S.
Scientist 1-301-816-8161 |
(MDANT05) Monograph Development-Antibiotics |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 2860
Pharmacopeial Forum: Volume No. 34(3) Page 627
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.