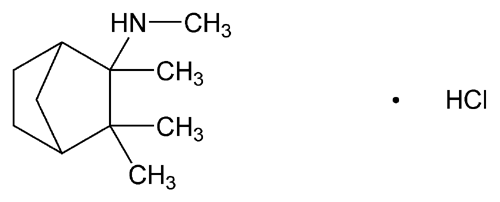
Mecamylamine Hydrochloride
Bicyclo[2.2.1]heptan-2-amine, N,2,3,3-tetramethyl-, hydrochloride.
N,2,3,3-Tetramethyl-2-norbornanamine hydrochloride
» Mecamylamine Hydrochloride contains not less than 98.0 percent and not more than 100.5 percent of C11H21N·HCl, calculated on the dried basis.
Packaging and storage—
Preserve in tight containers.
USP Reference standards  11
11 —
—
USP Mecamylamine Hydrochloride RS.
USP Mecamylamine Related Compound A RS .
.
USP Mecamylamine Hydrochloride RS.
USP Mecamylamine Related Compound A RS
 .
.
Acidity—
Dissolve 5.0 g in 100 mL of methanol, and titrate potentiometrically with 0.10 N alcoholic potassium hydroxide to an apparent pH of 5.5, using a calomel-glass electrode system and a potentiometer previously standardized with pH 5.0 neutralized phthalate buffer (see Solutions in the section Reagents, Indicators, and Solutions): after correction for the volume of alkali consumed by 100 mL of methanol, not more than 0.55 mL of 0.10 N alcoholic potassium hydroxide is required.
Loss on drying  731
731 —
Dry it at a pressure not exceeding 5 mm of mercury at 105
—
Dry it at a pressure not exceeding 5 mm of mercury at 105 for 1 hour: it loses not more than 1.0% of its weight.
for 1 hour: it loses not more than 1.0% of its weight.
Residue on ignition  281
281 :
not more than 0.5%.
:
not more than 0.5%.
Heavy metals, Method I  231
231 —
Dissolve 400 mg in 20 mL of water, add 2 mL of 1 N acetic acid, and dilute with water to 25 mL: the limit is not more than 50 ppm.
—
Dissolve 400 mg in 20 mL of water, add 2 mL of 1 N acetic acid, and dilute with water to 25 mL: the limit is not more than 50 ppm.
Limit of residual solvents—
Diluent—
Prepare a mixture of dimethyl sulfoxide and water (2:1).
Internal standard solution—
Prepare a solution of absolute alcohol in Diluent having a known concentration of about 15 µL per mL.
Standard stock solution—
Transfer 50 mL of the Diluent to a 100-mL volumetric flask, add 0.64 mL of isopropyl alcohol, dilute with Diluent to volume, and mix.
Standard solution—
Pipet 1.0 mL of the Standard stock solution into a 25-mL volumetric flask, dilute with Diluent to volume, and mix (Solution 1). Transfer about 500 mg of sodium chloride, accurately weighed, to a headspace vial, add 1.5 mL of Solution 1 and 1.5 mL of the Internal standard solution, and mix.
Test solution—
Transfer about 150 mg of Mecamylamine Hydrochloride, accurately weighed, to a headspace vial, add about 500 mg of sodium chloride, 1.5 mL of Diluent, and 1.5 mL of the Internal standard solution, and mix.
Chromatographic system (see Chromatography  621
621 )—
The gas chromatograph is equipped with a flame-ionization detector and a 0.53-mm × 30-m capillary column whose internal wall is coated with a 1.0-µm film of liquid phase G16. This column is joined with a 0.53-mm × 25-m capillary column whose internal wall is coated with a 5.0-µm film of liquid phase G1. The G16 column is connected to the detector, and the G1 column is connected to the injector. The injection port temperature is maintained at about 100
)—
The gas chromatograph is equipped with a flame-ionization detector and a 0.53-mm × 30-m capillary column whose internal wall is coated with a 1.0-µm film of liquid phase G16. This column is joined with a 0.53-mm × 25-m capillary column whose internal wall is coated with a 5.0-µm film of liquid phase G1. The G16 column is connected to the detector, and the G1 column is connected to the injector. The injection port temperature is maintained at about 100 ; the detector temperature is maintained at about 210
; the detector temperature is maintained at about 210 ; and the column temperature is maintained at 50
; and the column temperature is maintained at 50 for 10 minutes, then increased at a rate of 5
for 10 minutes, then increased at a rate of 5 per minute to 110
per minute to 110 , then increased at a rate of 30
, then increased at a rate of 30 per minute to 210
per minute to 210 , and maintained for 5 minutes at 210
, and maintained for 5 minutes at 210 . Nitrogen is used as the carrier gas, flowing at a rate of about 6.5 mL per minute. The split flow is 15 mL per minute.
. Nitrogen is used as the carrier gas, flowing at a rate of about 6.5 mL per minute. The split flow is 15 mL per minute.
Procedure—
Allow the Standard solution, the Internal standard solution, and the Test solution to stand for 20 minutes at 90 . Separately inject equal volumes (about 1 mL) of the headspace of the Standard solution, the Internal standard solution, and the Test solution into the gas chromatograph, record the chromatograms, and measure the peak responses of the internal standard and isopropyl alcohol. Calculate the quantity, in ppm, of isopropyl alcohol in the portion of Mecamylamine Hydrochloride taken by the formula:
. Separately inject equal volumes (about 1 mL) of the headspace of the Standard solution, the Internal standard solution, and the Test solution into the gas chromatograph, record the chromatograms, and measure the peak responses of the internal standard and isopropyl alcohol. Calculate the quantity, in ppm, of isopropyl alcohol in the portion of Mecamylamine Hydrochloride taken by the formula:
150(RU)(WS) / (RS)(WU)
in which WS is the amount, in ppm, of isopropyl alcohol in the Standard solution; WU is the weight, in mg, of Mecamylamine Hydrochloride taken to prepare the Test solution; and RU and RS are the peak response ratios of isopropyl alcohol to the internal standard obtained from the Test solution and the Standard solution, respectively: not more than 2000 ppm of isopropyl alcohol is found.
Related compounds—
Internal standard solution—
Proceed as directed in the Assay.
Solution 1—
Prepare a solution of dl-camphene and USP Mecamylamine Related Compound A RS in the Internal standard solution containing 625 µg of each per mL.
System suitability solution—
Transfer about 125 mg of USP Mecamylamine Hydrochloride RS, accurately weighed, to a 50-mL volumetric flask, add 1 mL of Solution 1, dilute with Internal standard solution to volume, and mix.
Test solution—
Use the Assay preparation.
Chromatographic system—
Prepare as directed in the Assay. Chromatograph the System suitability solution, and record peak responses as directed for Procedure: the resolution, R, between the mecamylamine and mecamylamine related compound A is not less than 5; the column efficiency is not less than 4000 theoretical plates; the tailing factor is not more than 1.5; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Inject a volume (about 1 µL) of the Test solution into the chromatograph, record the chromatogram, and measure all the peak responses. Calculate the percentage of each impurity in the portion of Mecamylamine Hydrochloride taken by the formula:
100(ri / rs)
in which ri is the peak response for each impurity; and rs is the sum of the responses for all the peaks: not more than 0.5% of mecamylamine related compound A is found; not more than 0.5% of dl-camphene is found; and not more than 1.0% of total impurities is found.
Chloride content—
Dissolve about 500 mg, accurately weighed, in 5 mL of water. Add 5 mL of glacial acetic acid, 50 mL of methanol, and 1 drop of eosin Y TS, and titrate with 0.1 N silver nitrate VS. Each mL of 0.1 N silver nitrate is equivalent to 3.545 mg of Cl: the content is between 17.0% and 17.8%.
Assay—
Internal standard solution—
Transfer about 600 mg of sodium hydroxide pellets to a 1 L volumetric flask, dissolve in about 800 mL of methanol. Add an accurately weighed quantity of about 1.7 g of biphenyl to the flask, and dilute with methanol to volume.
Standard preparation—
Dissolve an accurately weighed quantity of USP Mecamylamine Hydrochloride RS in Internal standard solution, and dilute with Internal standard solution, quantitatively and stepwise if necessary, to obtain a solution having a known concentration of about 2.5 mg per mL.
Assay preparation—
Transfer about 125 mg of Mecamylamine Hydrochloride, accurately weighed, to a 50-mL volumetric flask, dissolve in and dilute with Internal standard solution to volume, and mix.
Chromatographic system (see Chromatography  621
621 )—
The gas chromatograph is equipped with a flame-ionization detector connected to a 0.53-mm × 30-m capillary column, coated with a 1.5-µm film of liquid phase G27. The injection port temperature is maintained at about 200
)—
The gas chromatograph is equipped with a flame-ionization detector connected to a 0.53-mm × 30-m capillary column, coated with a 1.5-µm film of liquid phase G27. The injection port temperature is maintained at about 200 , the detector temperature is maintained at about 280
, the detector temperature is maintained at about 280 , and the column temperature is at 120
, and the column temperature is at 120 for 15 minutes then increased at 25
for 15 minutes then increased at 25 per minute to 250
per minute to 250 and maintained for 7 minutes at 250
and maintained for 7 minutes at 250 . Nitrogen is used as the carrier gas at 7.4 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the column efficiency is not less than 4000 theoretical plates; the tailing factor is not more than 1.5; and the relative standard deviation for replicate injections is not more than 2.0%.
. Nitrogen is used as the carrier gas at 7.4 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the column efficiency is not less than 4000 theoretical plates; the tailing factor is not more than 1.5; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Inject equal volumes (about 1 µL) of the Assay preparation and the Standard preparation into the gas chromatograph, record the chromatogram, and measure the responses for the major peaks. Calculate the quantity, in mg, of C11H21N·HCl in the portion of Mecamylamine Hydrochloride taken by the formula:
50C(RU / RS)
in which C is the concentration of USP Mecamylamine Hydrochloride RS, in mg per mL, in the Standard preparation; and RU and RS are the peak response ratios of mecamylamine hydrochloride to the internal standard biphenyl obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Sujatha Ramakrishna, Ph.D.
Scientist 1-301-816-8349 |
(MDCV05) Monograph Development-Cardiovascular |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 2855
Pharmacopeial Forum: Volume No. 28(6) Page 1817
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.