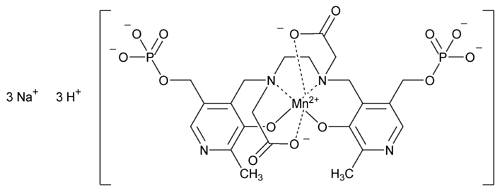
Mangafodipir Trisodium
C22H27MnN4Na3O14P2




 757.33
757.33
Trisodium trihydrogen (OC-6-13)-[[N,N¢-1,2-ethanediylbis[N-[[3-hydroxy-2-methyl-5-[(phosphonooxy)methyl]-4-pyridinyl]methyl]glycinato]](8-)] manganate(6-).
Trisodium trihydrogen (OC-6-13)-[[N,N¢-ethylenebis[N-[[3-hydroxy-5-(hydroxymethyl)-2-methyl-4-pyridyl]methyl]glycine] 5,5¢-bis(phosphato)](8-)]manganate(6-)

 [140678-14-4].
[140678-14-4].
Trisodium trihydrogen (OC-6-13)-[[N,N¢-1,2-ethanediylbis[N-[[3-hydroxy-2-methyl-5-[(phosphonooxy)methyl]-4-pyridinyl]methyl]glycinato]](8-)] manganate(6-).
Trisodium trihydrogen (OC-6-13)-[[N,N¢-ethylenebis[N-[[3-hydroxy-5-(hydroxymethyl)-2-methyl-4-pyridyl]methyl]glycine] 5,5¢-bis(phosphato)](8-)]manganate(6-)
» Mangafodipir Trisodium contains not less than 97.0 percent and not more than 103.0 percent of C22H27MnN4Na3O14P2, calculated on the anhydrous basis.
Packaging and storage—
Preserve in well-closed containers. Store in a cold place.
USP Reference standards  11
11 —
—
USP Endotoxin RS.
USP Mangafodipir Related Compound A RS .
.
USP Mangafodipir Related Compound B RS.
USP Mangafodipir Related Compound C RS .
.
USP Mangafodipir Trisodium RS .
.
USP Endotoxin RS.
USP Mangafodipir Related Compound A RS
 .
.
USP Mangafodipir Related Compound B RS.
USP Mangafodipir Related Compound C RS
 .
.
USP Mangafodipir Trisodium RS
 .
.
Identification—
Microbial enumeration tests  61
61 and Tests for specified microorganisms
and Tests for specified microorganisms  62
62 —
The total aerobic microbial count is not more than 500 cfu per g.
—
The total aerobic microbial count is not more than 500 cfu per g.
Bacterial endotoxins  85
85 :
not more than 0.13 USP Endotoxin Unit per mg.
:
not more than 0.13 USP Endotoxin Unit per mg.
pH  791
791 :
between 5.5 and 7.0, in a solution (1 in 100).
:
between 5.5 and 7.0, in a solution (1 in 100).
Water, Method I  921
921 :
not more than 20%.
:
not more than 20%.
Limit of residual solvents—
Internal standard solution—
Transfer 600 µL of methyl ethyl ketone to a 100-mL volumetric flask, dilute with water to volume, and mix to obtain a solution having a concentration of about 5 mg per mL. Transfer 2 mL of this solution to a 100-mL volumetric flask, dilute with water to volume, and mix to obtain a solution having a known concentration of about 0.1 mg per mL.
Standard stock solution—
Transfer about 1 g of dehydrated alcohol and 1 g of acetone, both accurately weighed, to a 100-mL volumetric flask, and dilute with water to volume. Transfer 10.0 mL of this solution to a 100-mL volumetric flask, dilute with water to volume, and mix to obtain a solution having a known concentration of about 1 mg each of alcohol and acetone per mL.
Standard solutions—
Transfer 10.0 mL of Internal standard solution to each of four 100-mL volumetric flasks. Separately add 0 mL, 1.0 mL, 5.0 mL, and 10.0 mL of Standard stock solution to the volumetric flasks, and dilute each with water to volume to obtain solutions having known concentrations of 0.0 µg per mL and about 10 µg per mL, 50 µg per mL, and 100 µg per mL each of alcohol and acetone, respectively. Add 7.0 mL of each Standard solution to separate headspace sample vials, and cap.
Test solution—
Transfer about 1 g of Mangafodipir Trisodium, accurately weighed, to a sample vial, add 7.0 mL of the Standard solution having a concentration of 0.0 µg per mL, cap, and swirl to dissolve.
Chromatographic system (see Chromatography  621
621 )—
The gas chromatograph is equipped with a flame-ionization detector, a 0.32-mm × 30-m fused silica column coated with 1.8-µm G43 stationary phase. The carrier gas is helium, flowing at a rate of 1.5 mL per minute. The temperatures of the injection port and the oven are maintained at 150
)—
The gas chromatograph is equipped with a flame-ionization detector, a 0.32-mm × 30-m fused silica column coated with 1.8-µm G43 stationary phase. The carrier gas is helium, flowing at a rate of 1.5 mL per minute. The temperatures of the injection port and the oven are maintained at 150 and 50
and 50 , respectively. The bath temperature for the headspace sample vials is maintained at 90
, respectively. The bath temperature for the headspace sample vials is maintained at 90 , the valve/loop temperature is maintained at 130
, the valve/loop temperature is maintained at 130 , and the sample thermostating time is 15 minutes. Chromatograph the Standard solutions, and record the peak responses as directed for Procedure: the resolution, R, between alcohol and acetone is not less than 5; and the relative standard deviation for replicate injections of the Standard solution having a concentration of 100 µg per mL, determined from the peak response ratios of the analyte to the internal standard, is not more than 2.0%. Calculate the peak response ratios of the analyte to the internal standard, and plot the results. Determine the linear regression equation of the standards by the mean-square method, and record the linear regression equation and the correlation coefficient. A suitable system is one that yields a line having a correlation coefficient of not less than 0.990.
, and the sample thermostating time is 15 minutes. Chromatograph the Standard solutions, and record the peak responses as directed for Procedure: the resolution, R, between alcohol and acetone is not less than 5; and the relative standard deviation for replicate injections of the Standard solution having a concentration of 100 µg per mL, determined from the peak response ratios of the analyte to the internal standard, is not more than 2.0%. Calculate the peak response ratios of the analyte to the internal standard, and plot the results. Determine the linear regression equation of the standards by the mean-square method, and record the linear regression equation and the correlation coefficient. A suitable system is one that yields a line having a correlation coefficient of not less than 0.990.
Procedure—
Separately inject equal volumes (about 1 mL) of the gaseous headspace of each of the Standard solutions and the Test solution into the chromatograph, record the chromatograms, and measure the peak responses. Calculate the percentages (w/w) of alcohol and acetone in the portion of Mangafodipir Trisodium taken by the formula:
(7/10,000)(C/W)
in which C is the concentration, in µg per mL, of alcohol or acetone in the Test solution, as determined from the relevant standard response line; and W is the weight, in g, of Mangafodipir Trisodium taken: not more than 0.1% of alcohol is found; and not more than 0.01% of acetone is found, both calculated on the anhydrous basis.
Limit of free manganese and free fodipir—
Ascorbic acid solution—
Dissolve 0.5 g of ascorbic acid in 10 mL of water.
Manganese solution—
Transfer about 3.6 g of manganese chloride, accurately weighed, to a 1000-mL volumetric flask, dissolve in and dilute with 0.1 N hydrochloric acid to volume, and mix. Transfer 100.0 mL of this solution to a 500-mL volumetric flask, dilute with water to volume, and mix.
Edetate titrant solution—
Transfer about 37 g of edetate disodium, accurately weighed, to a 1000-mL volumetric flask, dilute with water to volume, and mix. Transfer 36 mL of this solution to a 1000-mL volumetric flask, dilute with water to volume, and mix to obtain a solution having a concentration of 0.0036 moles per L.
standardization of 0.0036 m edetate titrant solution—
Accurately weigh about 200 mg of chelometric standard calcium carbonate, previously dried at 110 for 2 hours and cooled in a desiccator, transfer to a 100-mL volumetric flask, and add 10 mL of water and about 4 mL of diluted hydrochloric acid. Swirl the flask to dissolve, dilute with water to volume, and mix. Transfer 5.0 mL of this solution to a beaker while stirring, preferably with a magnetic stirrer; and add about 15 mL of sodium hydroxide TS and enough hydroxynaphthol blue indicator to achieve a percent transmission of about 95%, using a suitable autotitrator at a wavelength of 620 nm, calibrated to 100% transmission with water. Add 20.0 mL of Edetate titrant solution, and continue to titrate until 3 mL of titrant have been added beyond the sharp break point, as determined from the titration curve obtained by plotting relative transmittance versus volume, in mL, of titrant added. Determine the endpoint volume from the titration curve. The final titration volume is the sum of the endpoint volume and the 20.0 mL of Edetate titrant solution initially added. Calculate the molarity of the Edetate titrant solution by the formula:
for 2 hours and cooled in a desiccator, transfer to a 100-mL volumetric flask, and add 10 mL of water and about 4 mL of diluted hydrochloric acid. Swirl the flask to dissolve, dilute with water to volume, and mix. Transfer 5.0 mL of this solution to a beaker while stirring, preferably with a magnetic stirrer; and add about 15 mL of sodium hydroxide TS and enough hydroxynaphthol blue indicator to achieve a percent transmission of about 95%, using a suitable autotitrator at a wavelength of 620 nm, calibrated to 100% transmission with water. Add 20.0 mL of Edetate titrant solution, and continue to titrate until 3 mL of titrant have been added beyond the sharp break point, as determined from the titration curve obtained by plotting relative transmittance versus volume, in mL, of titrant added. Determine the endpoint volume from the titration curve. The final titration volume is the sum of the endpoint volume and the 20.0 mL of Edetate titrant solution initially added. Calculate the molarity of the Edetate titrant solution by the formula:
(5/100.09)(W)/(100V)
in which 100.09 is the molecular weight of calcium carbonate; W is the weight, in mg, of the calcium carbonate taken; and V is the final titration volume, in mL, of Edetate titrant solution.
Procedure—
Transfer about 1 g of Mangafodipir Trisodium, accurately weighed, to a suitable beaker, add about 100 mL of water, 1.0 mL of Ascorbic acid solution, 10 mL of ammonia–ammonium chloride buffer TS, 0.1 mL of eriochrome black TS, and 1.0 mL of Manganese solution, and record the color. If the color is yellow to green, add additional 1.0-mL increments of Manganese solution until the color is red. Record the volume added. Titrate with the Edetate titrant solution, determining the endpoint photometrically. Perform a blank determination, and make any necessary correction (see Titrimetry  541
541 ). Calculate the percentage of free manganese in the portion of Mangafodipir Trisodium taken by the formula:
). Calculate the percentage of free manganese in the portion of Mangafodipir Trisodium taken by the formula:
5.49V(M/W)
in which V is the volume, in mL, of the Edetate titrant solution; M is the molarity of the Edetate titrant solution; and W is the weight, in g, of Mangafodipir Trisodium taken. Calculate the percentage of free fodipir in the portion of Mangafodipir Trisodium taken by the formula:
63.85V(M/W)
in which V, M, and W are as defined herein: not more than 0.03% of free manganese is found; and not more than 0.5% of free fodipir is found, both calculated on the anhydrous basis.
Related compounds—
Ascorbic acid solution—
Dissolve 0.4 g of ascorbic acid in 100 mL of water.
Phosphate buffer—
Prepare as directed in the Assay.
Mobile phase—
Prepare as directed in the Assay. [note—Increasing the proportion of acetonitrile will decrease the retention times.]
System suitability stock solution—
Prepare as directed for Standard stock preparation in the Assay.
System suitability solution 1—
Prepare a solution of USP Mangafodipir Trisodium RS having a known concentration of about 4.0 mg per mL. Transfer 5.0 mL of this solution to a 50-mL volumetric flask, add 5.0 mL of System suitability stock solution, 5.0 mL of Phosphate buffer, and 5.0 mL of Ascorbic acid solution. Dilute with nitrogen-purged water to volume, and mix to obtain a solution having a concentration of about 0.4 mg of USP Mangafodipir Trisodium RS, and about 0.01 mg each of USP Mangafodipir Related Compound A RS and USP Mangafodipir Related Compound B RS per mL. [note—Store in a refrigerator and under nitrogen to avoid excessive exposure to heat, air, and light.]
System suitability solution 2—
Transfer about 10 mg of USP Mangafodipir Related Compound C RS to a 100-mL volumetric flask, dilute with water to volume, and mix. Transfer 5.0 mL of this solution to a 50-mL volumetric flask, and add 5.0 mL of Phosphate buffer.
Test solution—
Transfer an accurately weighed quantity of Mangafodipir Trisodium, equivalent to about 100 mg of mangafodipir trisodium, to a 50-mL volumetric flask, dilute with water to volume, and mix. Transfer 10.0 mL of this solution to a second 50-mL volumetric flask, add 5.0 mL of Phosphate buffer, dilute with water to volume, and mix. [note—Store in a refrigerator and under nitrogen to avoid excessive exposure to heat, air, and light.]
Chromatographic system (see Chromatography  621
621 )—
Prepare as directed in the Assay. Chromatograph System suitability solution 2, and record the peak responses as directed for Procedure: note the elution time to identify the mangafodipir related compound C peak, if present, in the chromatogram of System suitability solution 1. Chromatograph System suitability solution 1, and record the peak responses as directed for Procedure: the retention time for mangafodipir is between 18 and 30 minutes. The peak area for mangafodipir related compound C is less than 0.1%. [note—If the peak area is more than 0.1% of the total of all peak areas, prepare fresh quantities of Ascorbic acid solution and System suitability solution 1, and repeat the test. If the peak area of mangafodipir related compound C is still greater than 0.1%, repeat the test using another column. A contaminated column can result in oxidation of Mn(II) to Mn(III), forming related compound C.] The tailing factor for the mangafodipir peak is not more than 2.3; the column efficiency is not less than 1000 theoretical plates; the resolution, R, between mangafodipir related compound B and mangafodipir is not less than 1.5; and the relative standard deviation for replicate injections is not more than 10% for each peak. [note—If the resolution is less than 1.5, adjust the Mobile phase by increasing the concentration of tetrabutylammonium hydrogen sulfate.]
)—
Prepare as directed in the Assay. Chromatograph System suitability solution 2, and record the peak responses as directed for Procedure: note the elution time to identify the mangafodipir related compound C peak, if present, in the chromatogram of System suitability solution 1. Chromatograph System suitability solution 1, and record the peak responses as directed for Procedure: the retention time for mangafodipir is between 18 and 30 minutes. The peak area for mangafodipir related compound C is less than 0.1%. [note—If the peak area is more than 0.1% of the total of all peak areas, prepare fresh quantities of Ascorbic acid solution and System suitability solution 1, and repeat the test. If the peak area of mangafodipir related compound C is still greater than 0.1%, repeat the test using another column. A contaminated column can result in oxidation of Mn(II) to Mn(III), forming related compound C.] The tailing factor for the mangafodipir peak is not more than 2.3; the column efficiency is not less than 1000 theoretical plates; the resolution, R, between mangafodipir related compound B and mangafodipir is not less than 1.5; and the relative standard deviation for replicate injections is not more than 10% for each peak. [note—If the resolution is less than 1.5, adjust the Mobile phase by increasing the concentration of tetrabutylammonium hydrogen sulfate.]
Procedure—
Inject about 10 µL of the Test solution into the chromatograph, record the chromatogram, and measure the areas for all the major peaks. The relative retention times for ascorbic acid, mangafodipir related compound A, Mn(II)-5-methyl dipyridoxal monophosphate (Mn(II)-5-methyl DPMP) if present, mangafodipir related compound C, mangafodipir related compound B, and mangafodipir are about 0.1, 0.3, 0.4, 0.6, 0.8, and 1.0, respectively. Calculate the percentages of mangafodipir related compound A, mangafodipir related compound B, mangafodipir related compound C, and Mn(II)-5-methyl DPMP in the portion of Mangafodipir Trisodium taken by the formula:
100(ri / rs)
in which ri is the peak area of each impurity; and rs is the sum of the areas of all of the peaks: not more than 0.5% each of mangafodipir related compound A and mangafodipir related compound B is found; not more than 0.6% of mangafodipir related compound C is found; not more than 0.3% of Mn(II)-5-methyl DPMP is found; not more than 0.3% of any other impurity is found; not more than a total of 0.5% of other impurities is found; and not more than a total of 2.0% of impurities is found.
Assay—
Phosphate buffer—
Transfer about 26.8 g of dibasic sodium phosphate to a 1000-mL volumetric flask, add 900 mL of water, and adjust with 1 N sodium hydroxide or 1 N hydrochloric acid to a pH of about 8.0. Dilute with water to volume, filter, and degas.
Mobile phase—
Transfer about 0.61 g of boric acid and 9.2 g of tetrabutylammonium hydrogen sulfate to a 1000-mL volumetric flask, add 640 mL water, and mix. Adjust with 3 N sodium hydroxide to a pH of about 9.3, add 250 mL of acetonitrile, dilute with water to volume, and mix. Adjust with 3 N hydrochloric acid or 3 N sodium hydroxide to a pH of about 10.5, filter, and degas. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard stock preparation—
Transfer about 10 mg each of USP Mangafodipir Related Compound A RS and USP Mangafodipir Related Compound B RS, both accurately weighed, to a 100-mL volumetric flask, dilute with water to volume, and mix.
Standard preparation—
Transfer about 100 mg of USP Mangafodipir Trisodium RS to a 50-mL volumetric flask, dilute with water to volume, and mix. Transfer 10.0 mL of this solution to a 50-mL volumetric flask, add 5.0 mL of Standard stock preparation and 5.0 mL of Phosphate buffer, dilute with water to volume, and mix. [note—Store in a refrigerator and under nitrogen to avoid exposure to excessive heat, air, or light.]
Assay preparation—
Transfer an accurately measured quantity of Mangafodipir Trisodium, equivalent to about 100 mg of mangafodipir trisodium, to a 50-mL volumetric flask, dilute with water to volume, and mix. Transfer 10.0 mL of this solution to a 50-mL volumetric flask, add 5.0 mL of Phosphate buffer, dilute with water to volume, and mix. [note—Store in a refrigerator and under nitrogen to avoid exposure to excessive heat, air, or light.]
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 310-nm detector and a 4.6-mm × 15-cm column that contains 5-µm packing L21. The chromatograph is maintained at about 20
)—
The liquid chromatograph is equipped with a 310-nm detector and a 4.6-mm × 15-cm column that contains 5-µm packing L21. The chromatograph is maintained at about 20 . The flow rate is 0.8 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the resolution, R, between mangafodipir related compound A and mangafodipir related compound B is not less than 1.5; the column efficiency is not less than 1000 theoretical plates; and the tailing factor is not more than 2.3.
. The flow rate is 0.8 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the resolution, R, between mangafodipir related compound A and mangafodipir related compound B is not less than 1.5; the column efficiency is not less than 1000 theoretical plates; and the tailing factor is not more than 2.3.
Procedure—
Separately inject equal volumes (about 10 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the percentage of C22H27MnN4Na3O14P2 in the portion of Mangafodipir Trisodium taken by the formula:
25,000(C/W)(rU / rS)
in which C is the concentration, in mg per mL, of USP Mangafodipir Trisodium RS in the Standard preparation; W is the weight, in mg, of the Mangafodipir Trisodium taken; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Ian DeVeau, Ph.D.
Director, Veterinary Drugs and Radiopharmaceuticals 1-301-816-8178 |
(RMI05) Radiopharmaceuticals and Medical Imaging Agents 05 |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
|
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance | |
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance | |
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance |
USP32–NF27 Page 2842
Pharmacopeial Forum: Volume No. 31(6) Page 1650