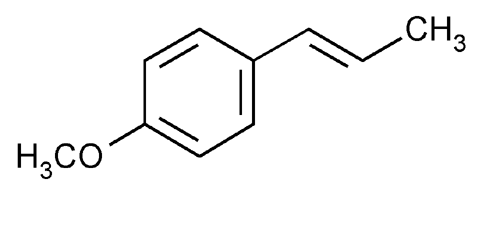
Anethole
Benzene, 1-methoxy-4-(1-propenyl)-, (E)-.
(E)-p-Propenylanisole
Synthetic
» Anethole is obtained from Anise Oil and other sources, or is prepared synthetically.
Packaging and storage—
Preserve in tight, light-resistant containers.
Labeling—
Label it to indicate whether it is obtained from natural sources or is prepared synthetically.
Specific gravity  841
841 :
between 0.983 and 0.988.
:
between 0.983 and 0.988.
Congealing temperature  651
651 :
not lower than 20
:
not lower than 20 .
.
Distilling range, Method I  721
721 :
between 231
:
between 231 and 237
and 237 , a correction factor of 0.063
, a correction factor of 0.063 per mm being applied as necessary.
per mm being applied as necessary.
Refractive index  831
831 :
between 1.557 and 1.561.
:
between 1.557 and 1.561.
Heavy metals, Method II  231
231 :
20 µg per g.
:
20 µg per g.
Aldehydes and ketones—
Shake 10 mL with 50 mL of a saturated solution of sodium bisulfite in a graduated cylinder, and allow the mixture to stand for 6 hours: no appreciable diminution in the volume of Anethole occurs, and no crystalline deposit separates.
Limit of phenols—
Shake 1 mL with 20 mL of water, and allow the liquids to separate. Pass the water layer through a filter paper previously moistened with water, and to 10 mL of the filtrate add 3 drops of ferric chloride TS: no purple or purplish color is produced.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Robert H. Lafaver, B.A.
Scientist 1-301-816-8335 |
(EM105) Excipient Monographs 1 |
USP32–NF27 Page 1166
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.