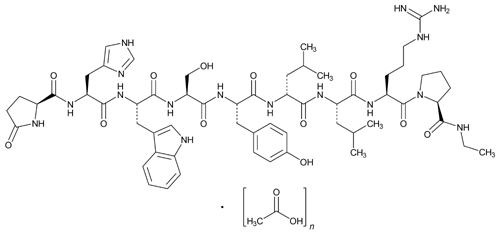
Leuprolide Acetate
C59H84N16O12·(C2H4O2)n, n = 1 or 2




 1209.41 (as free base)
1209.41 (as free base)
Luteinizing hormone-releasing factor, 6-d-leucine-9-(N-ethyl-l-prolinamide)-10-deglycinamide acetate (salt).
5-Oxo-l-prolyl-l-histidyl-l-tryptophyl-l-seryl-l-tyrosyl-d-leucyl-l-leucyl-l-arginyl-N-ethyl-l-prolinamide acetate (salt)

 [74381-53-6].
[74381-53-6].
Luteinizing hormone-releasing factor, 6-d-leucine-9-(N-ethyl-l-prolinamide)-10-deglycinamide acetate (salt).
5-Oxo-l-prolyl-l-histidyl-l-tryptophyl-l-seryl-l-tyrosyl-d-leucyl-l-leucyl-l-arginyl-N-ethyl-l-prolinamide acetate (salt)
» Leuprolide Acetate is a synthetic nonapeptide agonist analog of luteinizing hormone-releasing factor. It contains not less than 97.0 percent and not more than 103.0 percent of leuprolide (C59H84N16O12), calculated on the anhydrous, acetic acid-free basis.
note—Due to the hygroscopic nature of this material, analyses are performed immediately after opening the container in a glove box under dry nitrogen purge.
Caution—Leuprolide Acetate is a potent hormonal manipulator. Avoid skin contact and inhalation of dusts and mists.
Packaging and storage—
Preserve in tight containers. Store at a temperature not higher than 30 .
.
Identification—
B:
The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay.
Specific rotation  781S
781S :
between
:
between  38.0
38.0 and
and  42.0
42.0 expressed on an anhydrous, acetic acid-free basis.
expressed on an anhydrous, acetic acid-free basis.
Test solution:
10.0 mg per mL, in 1% acetic acid.
Bacterial endotoxins  85
85 —
It contains not more than 166.7 USP Endotoxin Units per mg of leuprolide acetate.
—
It contains not more than 166.7 USP Endotoxin Units per mg of leuprolide acetate.
Water, Method Ic  921
921 :
not more than 8.0%.
:
not more than 8.0%.
Residue on ignition  281
281 :
not more than 0.3%.
:
not more than 0.3%.
Chromatographic purity—
Buffer solution, Organic modifier solution, Mobile phase, Standard stock preparation, and Degradation standard preparation—
Prepare as directed in the Assay.
Standard solution—
Transfer 1.0 mL of the Standard stock preparation to a 100-mL volumetric flask, dissolve in and dilute with Mobile phase to volume, and mix.
Test solution—
Transfer about 100 mg of Leuprolide Acetate, accurately weighed, to a 100-mL volumetric flask, dissolve in and dilute with Mobile phase to volume, and mix.
Chromatographic system—
Proceed as directed in the Assay. Chromatograph the Test solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.80 for d-Ser-leuprolide, 0.90 for d-His-leuprolide, 1.00 for leuprolide, 1.2 for l-Leu6-leuprolide, and 1.5 for acetyl-leuprolide.
Procedure—
Separately inject equal volumes (about 20 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms for 90 minutes, and measure the peak responses. Calculate the percentage of each impurity in the portion of Leuprolide Acetate taken by the formula:
0.01(WS / WU)(ri / rS)P
in which WS is the weight of USP Leuprolide Acetate RS in the Standard stock preparation; WU is the weight, in mg, of Leuprolide Acetate in the Test solution; ri is the peak response for each impurity obtained from the Test solution; rS is the leuprolide peak response obtained from the Standard solution; and P is the designated purity, in percentage, of USP Leuprolide Acetate RS: not more than 1.0% of acetyl-leuprolide is found; not more than 0.5% each of d-His-leuprolide, l-Leu6-leuprolide, and d-Ser-leuprolide is found; not more than 0.5% of any other individual impurity is found; and not more than 2.5% of total impurities is found.
Content of acetic acid—
Diluent—
Use methanol, and adjust with phosphoric acid to a pH of 2.5.
Standard solution—
Pipet 2.0 mL of glacial acetic acid into a 100-mL volumetric flask, dilute with Diluent to volume, and mix. Transfer 4.0 mL of the solution so obtained to a 100-mL volumetric flask, dilute with Diluent to volume, and mix. Transfer 10.0 mL of this solution to a 100-mL volumetric flask, dilute with Diluent to volume, and mix to obtain a solution having a known concentration of about 0.08 mg per mL.
Test solution—
Transfer about 100 mg of Leuprolide Acetate, accurately weighed, to a 100-mL volumetric flask, dissolve in and dilute with Diluent to volume, and mix.
Chromatographic system (see Chromatography  621
621 )—
The gas chromatograph is equipped with a flame-ionization detector and a 0.53-mm × 30-m fused-silica capillary column that contains a 1.2-µm film of phase G35. The carrier gas is helium, flowing at a rate of about 10 mL per minute. The column temperature is maintained at about 100
)—
The gas chromatograph is equipped with a flame-ionization detector and a 0.53-mm × 30-m fused-silica capillary column that contains a 1.2-µm film of phase G35. The carrier gas is helium, flowing at a rate of about 10 mL per minute. The column temperature is maintained at about 100 , the injection port temperature is maintained at about 200
, the injection port temperature is maintained at about 200 , and the detector temperature is maintained at about 250
, and the detector temperature is maintained at about 250 . Chromatograph the Standard solution, and record the peak areas as directed for Procedure: the retention time of acetic acid is about 5 to 7 minutes; the column efficiency is not less than 15,000 theoretical plates; the tailing factor is not less than 0.8 and not more than 1.5; and the relative standard deviation for replicate injections is not more than 2.0%. Chromatograph the Diluent, and record the peak areas as directed for Procedure: verify that there are no interfering peaks.
. Chromatograph the Standard solution, and record the peak areas as directed for Procedure: the retention time of acetic acid is about 5 to 7 minutes; the column efficiency is not less than 15,000 theoretical plates; the tailing factor is not less than 0.8 and not more than 1.5; and the relative standard deviation for replicate injections is not more than 2.0%. Chromatograph the Diluent, and record the peak areas as directed for Procedure: verify that there are no interfering peaks.
Procedure—
Separately inject equal volumes (about 1.0 µL) of the Standard solution and the Test solution into the chromatograph (splitless mode), record the chromatograms, and measure the responses for the acetic acid peaks. Calculate the percentage of acetic acid (C2H4O2) in the portion of Leuprolide Acetate taken by the formula:
(839.2/WU)(rU / rS)
in which WU is the weight, in mg, of Leuprolide Acetate taken to prepare the Test solution; and rU and rS are the acetic acid peak areas obtained from the Test solution and the Standard solution, respectively: not less than 4.7% and not more than 9.0% is found.
Amino acid content—
Use a suitable, validated procedure (see Biotechnology-Derived Articles—Amino Acid Anaylysis  1052
1052 ).
).
Standard solutions—
Prepare a solution having known equimolar amounts of l-alanine, l-arginine, l-aspartic acid, l-glutamic acid, glycine, l-histidine, l-isoleucine, l-leucine, l-lysine, l-methionine, l-phenylalanine, l-proline, l-serine, l-threonine, l-tyrosine, and l-valine with half the equimolar amount of l-cystine. For the validation of the method, an appropriate internal standard, such as norleucine, is used. Prepare a separate, equimolar solution of l-tryptophan.
Test solution—
Transfer about 64 mg of Leuprolide Acetate, accurately weighed, to a suitable vessel, and dissolve in 1.0 mL of water. Transfer 0.10 mL of this solution to a vacuum hydrolysis tube, add 2.0 mL of 6 N hydrochloric acid, evacuate the tube, and heat for 16 hours at 120 . Transfer 0.10 mL of the hydrolysate so obtained to a suitable vessel, add 1 mL of water, and lyophilize. Dissolve in and dilute to a suitable volume in a buffer solution suitable for amino acid analysis.
. Transfer 0.10 mL of the hydrolysate so obtained to a suitable vessel, add 1 mL of water, and lyophilize. Dissolve in and dilute to a suitable volume in a buffer solution suitable for amino acid analysis.
Procedure—
Inject equal volumes of the Standard solutions and the Test solution into the amino acid analyzer, and record and measure the responses for each amino acid peak. Express the content of each amino acid in moles. Calculate the relative proportions of the amino acids in the Test solution, taking one-seventh of the sum of the number of moles of histidine, glutamic acid, leucine, proline, tyrosine, and arginine as equal to one: between 0.85 and 1.1 moles each of glutamic acid, proline, tyrosine, histidine, and arginine per mole of Leuprolide Acetate is found; between 1.8 and 2.2 moles of leucine per mole of Leuprolide Acetate is found. Serine and tryptophan are also present.
Assay—
Buffer solution—
Dissolve about 15.2 g of triethylamine in 800 mL of water, adjust with phosphoric acid to a pH of 3.0, and dilute with water to 1000 mL.
Organic modifier solution—
Prepare a mixture of acetonitrile and n-propyl alcohol (3:2).
Mobile phase—
Prepare a filtered and degassed mixture of Buffer solution and Organic modifier solution (85:15). Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard stock preparation—
Transfer about 100 mg of USP Leuprolide Acetate RS, accurately weighed, to a 100-mL volumetric flask, dissolve in and dilute with Mobile phase to volume, and mix.
Standard preparation—
Transfer 5.0 mL of the Standard stock preparation into a 100-mL volumetric flask, dissolve in and dilute with Mobile phase to volume, and mix.
Degradation standard preparation—
Transfer 5 mL of the Standard stock preparation to a 50-mL volumetric flask, and dilute with water to volume. Transfer 5 mL of the solution so obtained to a scintillation vial. Add 100 µL of 1 N sodium hydroxide solution, tightly cap, and shake vigorously. Place in an oven at a 100 for 60 minutes, remove, allow to cool, add 50 µL of 1 M phosphoric acid, recap, and shake vigorously to mix.
for 60 minutes, remove, allow to cool, add 50 µL of 1 M phosphoric acid, recap, and shake vigorously to mix.
Assay preparation—
Transfer about 100 mg of Leuprolide Acetate, accurately weighed, to a 100-mL volumetric flask, dissolve in and dilute with Mobile phase to volume, and mix. Transfer 5.0 mL of the solution so obtained to a 100-mL volumetric flask, dilute with Mobile phase to volume, and mix.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 220-nm detector and a 4.6-mm × 10-cm column that contains 3-µm packing L1. The flow rate is between 1.0 and 1.5 mL per minute. Chromatograph the Degradation standard preparation, and record the peak responses as directed for Procedure: the retention time of leuprolide is between 41 and 49 minutes; the relative retention times are about 0.90 for the degradation product and 1.0 for leuprolide; and the resolution, R, between leuprolide and the degradation product is not less than 1.5. Chromatograph the Mobile phase, and record the peak responses as directed for Procedure: verify that no extraneous peaks are present. Chromatograph the Standard preparation, and record the peak areas as directed for Procedure: the tailing factor is not less than 0.8 and not more than 1.5; and the relative standard deviation for replicate injections is not more than 1.5%.
)—
The liquid chromatograph is equipped with a 220-nm detector and a 4.6-mm × 10-cm column that contains 3-µm packing L1. The flow rate is between 1.0 and 1.5 mL per minute. Chromatograph the Degradation standard preparation, and record the peak responses as directed for Procedure: the retention time of leuprolide is between 41 and 49 minutes; the relative retention times are about 0.90 for the degradation product and 1.0 for leuprolide; and the resolution, R, between leuprolide and the degradation product is not less than 1.5. Chromatograph the Mobile phase, and record the peak responses as directed for Procedure: verify that no extraneous peaks are present. Chromatograph the Standard preparation, and record the peak areas as directed for Procedure: the tailing factor is not less than 0.8 and not more than 1.5; and the relative standard deviation for replicate injections is not more than 1.5%.
Procedure—
Separately inject equal volumes (about 20 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms for 60 minutes, and measure the leuprolide peak areas. Calculate the percentage of leuprolide (C59H84N16O12) in the portion of Leuprolide Acetate taken by the formula:
[(WS / WU)(rU / rS)(P)(0.9527)(100)] /(100 – acetic acid content – water content)
in which WS is the weight, in mg, of USP Leuprolide Acetate RS in the Standard preparation; WU is the weight, in mg, of Leuprolide Acetate in the Assay preparation; rU and rS are the peak areas obtained from the Assay preparation and the Standard preparation, respectively; and P is the designated purity, in percentage, of USP Leuprolide Acetate RS.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Larry N. Callahan, Ph.D.
Senior Scientist 1-301-816-8385 |
(BBPP05) Biologics and Biotechnology - Proteins and Polysaccharides |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
|
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance |
USP32–NF27 Page 2761
Pharmacopeial Forum: Volume No. 30(3) Page 882